Uncategorized
Guest post by Alain Braillon
I am not sure to understand the aim of the publication in the BMJ about traditional medicine entitled “India’s struggle to integrate traditional medicine into modern healthcare”.(1)
Indeed, not only it uncritically reported the establishment of the WHO Global Traditional Medicine Centre in Gujarat (1) but it also by-passed that diarrhea is a leading cause of child mortality in India, as in many poor countries. Oral rehydration salts are an inexpensive and lifesaving treatment for child diarrhea but they are widely underused.(2) This hardly acceptable and understandable state of affairs is driven by perceptions that patients do not want oral rehydration salts.(2) Obviously, the perceptions that healthcare professionals must provide culturally appropriate care can have devastating consequences.
Furthemore the case is also a political one with corruption. How could Krishna and the BMJ’s reviewers ignore a decision of the Supreme Court of India?(3) The Court has temporarily banned Patanjali Ayurved – named after a Hindu mystic best known for his writings on yoga – from advertising some of its traditional ayurvedic products.
India’s ruling party is the BJP and Prime Minister Narendra Modi glorifies Hindu traditions. Modi inaugurated Patanjali’s ayurvedic research facility in 2017. Baba Ramdev, a guru and the “marketing ambassador” of Patanjali’s business had received an estimated $46 million in discounts for land acquisitions in states controlled by the BJP.(4)
In 1861, Cheever warned in the journal that is now the New England Journal of Medicine that although “savages” could have great powers of observation, they had no understanding of disease or therapeutic mechanisms that require rigorous analysis of facts with the avoidance of biases.(5) Obviously, modern medicine that required strong evidence, not the subjective experience of emotions, is not a recent concept. Accordingly, I wonder if Krishna could have confused “modern” with “contemporary”. Indeed, presently healthcare is less and less about evidence for a positive benefit/harm ratio on relevant clinical outcomes, as illustrated by the growing flow of marketing approvals based on unvalidated surrogates(6,7) an, and more and more about fraudulent marketing.( 8,9)
Happily, medical devices of our traditional medicine, lancets for bloodletting and clysters for enema, are relegated to museums.
References
- Krishna G. India’s struggle to integrate traditional medicine into modern healthcare. BMJ. 2024;384:q268.5. doi:10.1136/bmj.q268
- Wagner Z, Mohanan M, Zutshi R, Mukherji A, Sood N. What drives poor quality of care for child diarrhea? Experimental evidence from India. Science. 2024;383(6683):eadj9986. doi:10.1126/science.adj9986
- https://www.npr.org/sections/goatsandsoda/2024/03/14/1236533011/ayurvedic-india-banned-advertising-some-products
- https://www.reuters.com/investigates/special-report/india-modi-ramdev/
- Cheever DW. The value and the fallacy of statistics in the observation of disease. Boston Med Surg J 1861;63:476-483 doi:10.1056/NEJM186101100632402
- Brinkhuis F, Goettsch WG, Mantel-Teeuwisse AK, Bloem LT. Added benefit and revenues of oncology drugs approved by the European Medicines Agency between 1995 and 2020: retrospective cohort study. BMJ. 2024;384:e077391. doi:10.1136/bmj-2023-077391
Proponents of so-called alternative medicine (SCAM) are often – as we had many opportunities to observe here on this blog – not impressed with the safety and efficacy of COVID vaccinations. This is despite the fact that several studies have demonstrated the huge number of lives saved by them, both at national and multi-country level in the earlier stages of the pandemic. I wonder whether the doubters will be convinced by new evidence.
This analysis estimates how many lives were directly saved by vaccinating adults against COVID in the Region, from December 2020 through March 2023.
The researchers estimated the number of lives directly saved by age-group, vaccine dose and circulating Variant of Concern (VOC) period, both regionally and nationally, using weekly data on COVID-19 mortality and COVID-19 vaccine uptake reported by 34 European areas and territories (CAT), and vaccine effectiveness (VE) data from the literature. They calculated the percentage reduction in the number of expected and reported deaths.
The authors found that vaccines reduced deaths by 57% overall (CAT range: 15% to 75%), representing ∼1.4 million lives saved in those aged ≥25 years (range: 0.7 million to 2.6 million): 96% of lives saved were aged ≥60 years and 52% were aged ≥80 years; first boosters saved 51%, and 67% were saved during the Omicron period.
The authors concluded that over nearly 2.5 years, most lives saved by COVID-19 vaccination were in older adults by first booster dose and during the Omicron period, reinforcing the importance of up-to-date vaccination among these most at-risk individuals. Further modelling work should evaluate indirect effects of vaccination and public health and social measures.
The authors feel that their results reinforce the importance of up-to-date COVID-19 vaccination, particularly among older age-groups. Communication campaigns supporting COVID-19 vaccination should stress the value of COVID-19 vaccination in saving lives to ensure vulnerable groups are up-to-date with vaccination ahead of periods of potential increased transmission.
Those SCAM proponents who are not convinced of the merits of COVID and other vaccinations will undoubtedly claim that this new analysis was biased and thus unreliable. Therefore, it seems worth stating that this work was supported by a US Centers for Disease Control cooperative agreement, who had no role in data analysis or interpretation. The authors affiliated with the World Health Organization (WHO) are alone responsible for the views expressed in this publication and they do not necessarily represent the decisions or policies of the WHO.
Like ultra-processed food (UPF) itself, the subject of UPF is everywhere – radio, TV, Twitter, you name it, the topic crops up. I too could not resist writing a post on it a few months ago. And now I am publishing another one but one in a slightly more irritated mood.
Why do these endless discussions on UPF irritate me?
To start with, there is no uniform definition of UPF, and many commentators seem more than a little confused about what UPF actually is. One definition holds that Ultra-processed foods are foods that have been altered to include fats, starches, sugars, salts and hydrogenated oils extracted from other foods. They contain ingredients, additives, and preservatives that are not normally used in home cooking. It seems obvious that discussions on UPF without a clear and understandable definition of the term are merely generating confusion in the general public.
But there are, of course, lists of UPF that might render the subject a bit clearer. The trouble, however, is that these lists reveal disagreement among each other. Thus they are prone to generate even more confusion.
Next, there is the evidence – and there is lots of it. It suggests that the regular consumption of UPF is bad for virtually every aspect of health. And if there is no evidence that it is detrimental for a given condition, it probably is merely because nobody has yet bothered to do the analyses. The trouble is, however, that all the relevant research comes from either basic science or epidemiology. This means that causality is unproven.
A further problem is that even the experts don’t know what the alleged causal factors in UPF are.
- Is it the processing?
- The additives?
- The sugar?
- The fats?
- If so, which fats exactly?
- Is it perhaps a complex inter-play of some of these factors?
If we want to make progress, we need to know! If not, we cannot possibly begin to avoid the health-threatening effects of UPF.
The final and arguably biggest problem is that UPF is everywhere. Nobody living in an industrialized country and earning a regular living can avoid consuming UPF. This means, I fear, that all the current hype about UPF is not just irritating but possibly counter-productive.
Imagine an average person trying to make sense of these discussions. She would soon give up and conclude that all these ‘clever’ experts know nothing at all. Her foremost concern is to make ends meet. In the end, she will carry on as before. Alternatively, she might even conclude that, as the even experts do not make sense, UPF cannot be all that bad after all.
After reading about and listening to the arguments around UPF, I ask myself this: would it not be more productive to apply more common sense and focus on a few nutritional messages that are 1) solidly based on evidence and 2) an average person can actually follow?
Traute Lafrenz Page was the last survivor of a non-violent resistance movement in Nazi Germany. On 6 March 2023, she died of natural causes at her farm in Yonges Island, S.C. aged 103.
Born Traute Lafrenz in Hamburg, Germany, Traute was a medical student in Munich when she became acquainted with other medical students like the Scholl siblings who opposed the Nazi regime. She and Hans Scholl were briefly romantically involved. Together they formed the Weisse Rose (White Rose). The students and one professor at the University of Munich (Willi Graf, Kurt Huber, Christoph Probst, Alexander Schmorell, Hans Scholl, and Sophie Scholl) began producing and distributing leaflets urging Germans to rise up in peaceful opposition to the Nazis. “Out of their readings of great writers evolved the initial idea about putting together these pamphlets that were focused on not only rebuking the Nazis but also invoking great names in literature and philosophy, and also rebuking the German people for not standing up,” Lafrenz Page’s daughter said.
Hans and Sophie Scholl, were arrested in 1943, convicted of high treason, and executed. Lafrenz Page attended their funerals and soon was arrested on charges of association. During her interrogation by the Gestapo, Lafrenz Page succeeded in disguising the full extent of her involvement in the distribution of leaflets. She was sentenced to serve a year in prison. After her release was arrested again by the Gestapo and faced trial in April 1945. Her life was spared as an advancing US Army liberated the prison just days before her trial was to begin.
“She knew the date of that trial for a long time, maybe even a year before it came up,” Lafrenz Page’s daughter said. “So what carried through the time of imprisonment and these experiences was the idea that the human spirit needed protection.”
After the war, Lafrenz Page completed her medical studies and moved to the US, where she served a medical residency in San Francisco and met her future husband, Vernon Page. He was an ophthalmologist who ran a medical practice in Hayfork, California, where she then practiced as a general practice physician. Traute hardly ever spoke about her involvement in the resistance against the Nazi regime and is quoted as saying; “Every complaint is forbidden in view of the fate of the others.”
Later, Lafrenz Page spent a year learning how to care for developmentally delayed children in Switzerland and became an enthusiast of Steiner’s anthroposophy. She began working as director of the Esperanza School in 1972. “She loved that work,” her daughter said. “On one level she just liked working with children, and on Marshfield, they also worked to address a child’s spirit, even if that child was handicapped or debilitated.”
In 2019, Lafrenz Page received the Order of Merit of the Federal Republic of Germany on the occasion of her 100th birthday. Her only Medline-listed article might provide some insight into her motivation in looking after disabled children:
This article examines the actions and testimonies of 14 nurses who killed psychiatric patients at the state hospital of Meseritz-Obrawalde in the Nazi ‘euthanasia’ program. The nurses provided various reasons for their decisions to participate in the killings. An ethical analysis of the testimonies demonstrates that a belief in the relief of suffering, the notion that the patients would ‘benefit’ from death, their selection by physicians for the ‘treatment’ of ‘euthanasia’, and a perceived duty to obey unquestioningly the orders of physicians were the primary ethical reasons that were stated for their behavior. However, 20 years had elapsed between the killings and the trial, thus giving ample opportunity for the defendants to develop comfortable rationales for their actions and for their attorneys to have observed successful defenses of others accused of euthanasia.
Acupuncture is questionable.
Acupressure is highly questionable.
Auricular acupressure is extremely questionable.
This study investigated the effect of auricular acupressure on the severity of postpartum blues. A randomized sham-controlled trial was conducted from February to November 2021, with 74 participants who were randomly allocated into two groups of either routine care + auricular acupressure (n = 37), or routine care + sham control (n = 37). Vacaria seeds with special non-latex adhesives were used to perform auricular acupressure on seven ear acupoints. There were two intervention sessions with an interval of five days. In the sham group, special non-latex adhesives without vacaria seeds were attached in the same acupoints as the intervention group. The severity of postpartum blues, fatigue, maternal-infant attachment, and postpartum depression was assessed. 
Auricular acupressure was associated with a significant effect in the reduction of postpartum blues on the 10th and 15th days after childbirth (SMD = −2.77 and −2.15 respectively), postpartum depression on the 21st day after childbirth (SMD = −0.74), and maternal fatigue on 10th, 15th and 21st days after childbirth (SMD = −2.07, −1.30 and −1.32, respectively). Also, the maternal-infant attachment was increased significantly on the 21st day after childbirth (SMD = 1.95).
The authors concluded that auricular acupressure was effective in reducing postpartum blues and depression, reducing maternal fatigue, and increasing maternal-infant attachment in the short-term after childbirth.
Let me put my doubts about these conclusions in the form of a few questions:
- If you had sticky tape on your ear, would you sometimes touch it?
- If you touched it, would you feel whether a vacaria seed was contained in it or not?
- Would you, therefore, say that such a trial could be properly blinded (not to forget the therapists who were, of course, in the know)?
- If the trial was thus de-blinded, would you claim that patient expectation did not influence the outcomes?
If you answered all of these questions with NO, you are – like I – of the opinion that the results of this trial could have easily been brought about, not by the alleged effects of acupressure, but by placebo and other non-specific effects.
Helmut Pilhar died in Paraguay on 31. August 2022. He was a prominent proponent of the ‘Germanische Heilkunde’, New German Medicine, a mixture of dangerous quackery and anti-semitism. The website of the ‘Germanische Heilkunde’ announced Pilhar’s death as follows: 
In light of this dramatic news, we would like to express our gratitude to Helmut Pilhar for his many years of work in spreading Germanic medicine.
Although our relationship with Helmut has been turbulent and unhappy in the time following Dr. Hamer’s departure, we will cherish the good times; and may the energy of warm appreciation from us reach him wherever he is.
A hug, Helmut.
Bona Hamer
We were saddened to learn that Helmut Pilhar passed from this life on August 31, 2022. For a long time he rendered valuable services to Germanic medicine and brought Dr. Hamer’s discovery to many people despite enormous opposition. For this he deserves our respect.
We regret his early death very much, our sympathy goes to his family.
Working Group of Germanic Medicine
Pilhar had no medical qualifications. The Wiesbadener Tagblatt dealt with Pilhar on November 5, 2013, calling him “the loudspeaker of madness”. The electrical engineer from Austria marketed the New German Medicine (NGM) full-time. He gave lectures and organized seminars in the whole German-speaking area and beyond.
Pilhar drew attention to himself in 1995 when he brought his five-year-old daughter Olivia, who had cancer, to the NGM inventor, Hamer, thus depriving her of effective therapy. The child was eventually operated on against her parents’ wishes and is now living in good health. While the media took great interest in the “Olivia case” and an unbearable hype arose around the child’s suffering, Helmut Pilhar sold photos of his daughter to newspapers and tried to influence the reporting. The couple received 500,000 shillings for the granting of broadcasting rights. The parents were sentenced to eight months probation. For Hamer, the unsuccessful NGM therapy attempt had no criminal consequences, as he was meanwhile working from Spain outside of Austria and Germany.
The last known places of residence of Helmut Pilhar were in Paraguay in the German-speaking “private colony El Paraíso Verde” near Cáazapa in Paraguay. According to his own statements, there he would not have to witness chemtrail.
Earlier this year, I started the ‘WORST PAPER OF 2022 COMPETITION’. You will ask: what is there to win in this competition? I agree: a competition without a prize is no fun. Therefore, I suggest offering the winner (that is the author of the winning paper) one of my books that best fits his/her subject. I am sure this will overjoy him or her. And how do we identify the winner? I will I continue blogging about nominated papers (I hope to identify about 10 in total), and towards the end of the year, I let my readers decide democratically.
In this spirit of democratic voting, let me suggest to you ENTRY No 7:
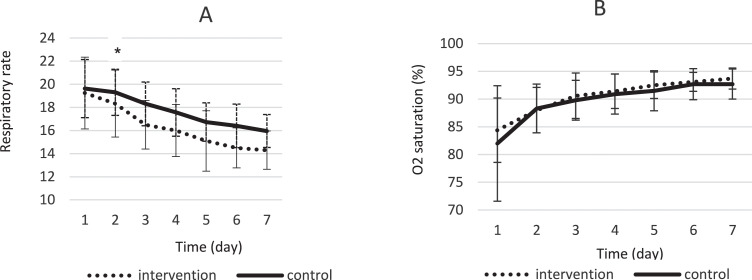
This trial evaluated the efficacy and safety of a Persian herbal medicine formula on patients with COVID-19. It was conducted in Afzalipour hospital, Kerman, Iran. Hospitalized COVID-19 patients were randomly divided into intervention (Persian herbal medicine formula + routine treatment) or control (only standard treatment) groups. The intervention group received capsule number 1 and 2 every 8 hours for 7 days. Capsule number 1 contained an extract of the Glycyrrhiza glabra, Punica granatum, and Rheum palmatum, and capsule number 2 was filled with Nigella sativa powder. Participants were followed up for 7 days. The primary outcome was the number of hospitalization days, while cough, fever, respiratory rate, days on oxygen (O2) therapy, and mortality rate were considered as secondary outcomes.
Eighty-two patients were enrolled in the study, while 79 cases completed the trial and their data were analyzed. The Persian medicine formula decreased the mean hospitalization days, so that the mean difference in length of hospitalization was 2.95 ± 0.43 days. A significant clinical improvement was observed regarding dyspnea, need for O2) therapy, and respiratory rate in the intervention group. Fever severity, cough severity, and death percentage were not statistically significantly different between groups. No adverse effects were reported.
The authors concluded that the present study supported the use of intended Persian medicine formulas as a supportive treatment for the hospitalized COVID-19 patients in order to improve their sign and symptoms, accelerate treatment, and shorten their hospitalization day during the disease period. It is recommended to conduct more clinical studies with bigger sample size for better evaluation.
This study begs a few questions:
- Why did the researchers not use a placebo in the control group?
- Why did they include only 82 patients in their study?
- Why did they not include outcomes that were independent of subjective influences?
- Why was mortality mentioned but no data provided?
- How is it possible that, under the conditions of the study, no adverse effects were noted?
- Why did you not stress the strong possibility that the effects are entirely due to placebo responses and wishful thinking?
- Why did you publish your study in a 3rd class journal?
I also have a few questions specifically for the editor of the Elsevier journal Integr Med Res, who decided to publish this study:
- Why did nobody bother to correct the poor English of the authors?
- Do you consider it ethical to publish such a poor-quality study?
- Are you not afraid that the conclusions might send many desperate patients up the garden path?
We are still in a pandemic where hundreds of people die every day. This means we are all extremely keen to find effective treatments. But it also means that an important ethical duty rests on all concerned to do rigorous research and abstain from unwarranted conclusions that might mislead us all.
The way I see it, there are two this paper can cause harm:
- Serious researchers will be put off from further investigating the herbal treatment. If the therapy is indeed effective, this would mean that progress is being hindered.
- More likely the treatment is useless. In this case, this study might cause considerable harm by prompting people to rely on an ineffective therapy.
For more than seven months, the Austrian general practitioner, Dr. Lisa-Maria Kellermayr, received death threats and was verbally attacked by the anti-vaccination cult. She eventually closed her practice, no longer wanted to leave the house, and apparently lost the courage to live. Last Friday, she was found dead in her practice in Seewalchen on Attersee. The public prosecutor’s office in Wels confirmed that there were no signs of foul play. Letters of farewell had been found. It is generally assumed that, in her desperation, Kellermayr took her own life. A friend tweeted: “In the end, she begged for help – from politicians, from the police, from her medical association. It never came. The police and the medical association would mock her. They said Dr Kellermayrjust wants to seek the limelight. Politicians ignored her. Now she is dead.”
The GP had closed her practice at the end of June, only temporarily, she said initially, because she could no longer afford the costs for her security, including a bodyguard. She did not feel sufficiently protected by the police. “For more than 7 months, we have been receiving death threats from the Covid measure opponents and vaccination opponents scene at irregular intervals,” Kellermayr tweeted. Threats went as far as predicting a “massacre” in her practice. In one of the threatening letters, the author described in detail how he would first torture and then murder the doctor and her practice staff.
At the end of July, she closed her practice for good. For the doctor, who loved her profession, it was a terrible decision. “I put so much money and energy into it,” she told ‘Der Spiegel’ just one day before her death. By her own account, she had at that stage invested around 100,000 Euros in security measures.
It is claimed that Kellermayr was badly let down by the Austrian authorities. On Austrian radio, the police even stated that Kellermayr merely sought the limelight to further her career. Now an official complaint has been filed charging the authorities with neglect.
Unbelievably, the despicable agitation of the anti-vaccination cult continues even after Kellermayr’s death. Most of the agitators remain anonymous. But some are also agitating publicly, for instance, on Twitter: Harald Laatsch, an AfD member of parliament in Berlin, wrote that the doctor was a vaccination propagandist and probably “no longer wanted to live with the heavy guilt”.
After Kellermayr’s suicide, well-known German Twitter users are withdrawing from the platform. The Würzburg lawyer Chan-jo Jun turned his back on Twitter last Friday stating: “If I tweet about certain people, I get warnings in my letterbox the next day. I am reaching my limits. I’m now leaving the field to others. Until I find a concept of how an objective exchange can take place in social media.”
Doctor Natalie Grams, a well-known critic of homeopathy, is also closing her Twitter account. She plans to continue her commitment to evidence-based medicine with the help of her podcast, Grams explained in her farewell tweet.
Others reached a different conclusion. Dr. Christian Luebbers tweeted this (my translation): “Of course, I also considered deactivating my account here. However, I came to the conclusion not to do so and continue to educate people about pseudo-medicine and vaccination. I see this as necessary moral courage and will not leave the field to hate.” Personally, I agree with him.
I would like to use the tragic occasion of Dr. Kellermyr’s death as an opportunity for making a plea. It is high time that intolerance, aggression, violence, and hatred stop. Please, let us all calm down and discuss with dignity and respect whatever issues we might have. Please, let this terrible death remind us that we are all human beings. Please, let it be a lesson to all of us.
The DAILY EXPRESS (DE) is not my favorite newspaper – perhaps even the opposite. During the last years, I have often been questioned by journalists on matters relating to so-called alternative medicine (SCAM). I do not recall, however, being interviewed by the DE (I might have forgotten, of course, but it certainly did not happen very often). I was therefore surprised to find that, in the last 13 years (this is as far back as I was able to search), the DE quoted me 22 times. Therefore, I decided to do a quick analysis of these 22 articles rating them (generously) for accuracy on a scale of 0 (totally inaccurate) to 10 (totally accurate).
1. Title (date of publication): Tracking down the safe alternatives (25 March 2008)
Subject: a new regulatory body (the Complementary and Natural Healthcare Council (CNHC)) might help separate the cranks from the credible.
Quote: The CNHC has been described as complementary medicine’s equivalent of the General Medical Council – the body which sets standards for GPs. It will investigate complaints and therapists who fall below expected standards could be struck off. The new organisation has been set up by Prince Charles’s Foundation for Integrated Health and receives part funding from the NHS. The Prince, who is a fan of homeopathy, believes that complementary therapies should have a greater role within the NHS…
Edzard Ernst, the UK’s first professor of complementary medicine, is scathing, describing the £2million cost of founding the CNHC as a waste of money. He says the new body does not challenge the safety or effectiveness of the therapies. “This organisation could give the public false confidence. Some of these therapies can do more harm than good. It will give them a status they don’t deserve.”
My comment (score): The subject matter was relevant. The article seems correct and my comment is true; the CNHC did, in fact, turn out to be a waste of space. (10)
2. Title (date of publication): Chinese Medicine: A risky remedy? (19 May 2008)
Subject: How much do we really know about how they work and could they actually be harmful to our health?
Quote: Traditional Chinese Medicine (TCM) is enjoying a boom with hundreds of shops appearing on high streets. The herbal medicine industry, which includes Chinese medicines, is worth an estimated £200million in the UK as thousands place their faith in ancient remedies for everything from acne to infertility…
Edzard Ernst, professor of complementary medicine at the University of Exeter and co-author of the book Trick Or Treatment: Alternative Medicine On Trial, says: “People think that because something is ancient or natural it must be good. That’s simply not true. Plenty of these medicines have side effects and can be dangerous. “TCMs are grossly under-researched in the UK. China’s research is hard to access and hard to understand. TCMs are frequently contaminated with toxic heavy metals. “This is because of poor quality, because soil is contaminated and supplying procedures are unregulated. The most worrying thing about TCMs is that they are regularly found to contain synthetic prescription drugs, which in extreme cases, taken wrongly, can kill.”
My comment (score): The subject matter was relevant. The article seems correct and my comment is true. (10)
3. Title (date of publication): Alternative treatments face calls for regulation (17 June 2008)
Subject: Alternative medicines must be regulated to protect patients from harm, according to an influential group of experts.
Quote: A government-appointed steering group said it was ridiculous that eight years after regulation was first called for, nothing had been done. And in a report to UK ministers, who have reserved powers on regulating health professionals, they warned it must be introduced “without delay”…
Prof Edzard Ernst, professor of complementary medicine at the University of Exeter, said there was no scientific evidence that homoeopathy works. Homoeopathy is the treatment of disease using minute doses of drugs diluted in water. Prof Ernst and author Simon Singh have pledged to give £10,000 to anyone who could prove, in a scientific way, that these treatments work as well as conventional medicines.
My comment (score): The subject matter was relevant. The article seems a bit confused. My comment seems to be from elsewhere and is out of context. (5)
4. Title (date of publication): Thank you for the music (28 June 2008)
Subject: Nerve disorder fibromyalgia left musician Emily Maguire housebound and in constant pain. As she prepares to play the Glastonbury festival she tells ABIGAIL JACKSON how her love of music pulled her through…
Quote: Dr Peter Fisher from the Royal London Homeopathic Hospital … has claimed to have success in treating fibromyalgia patients with homeopathic remedies. He prescribed ignacia, used as a remedy for numerous complaints from depression and sleeplessness to backache. A month later, Emily says the pain was gone. “I couldn’t believe it,” she says. “I feel so blessed.”
Although Emily is confident that taking ignacia (as well as maintaining a healthy lifestyle) did the trick, there are growing concerns over whether homeopathic remedies have any effect. Last week Edzard Ernst, the UK’s only professor of complementary medicine, offered £10,000 for any proof of a successful homeopathic treatment.
My comment (score): The subject matter is basically a case report which is not very relevant. The article seems confused and goes from the positive effects of music to homeopathy. What the article reports about our £10, 000 challenge is not relevant. (3)
5. Title (date of publication): Charles hit by ‘dodgy’ detox quackery row (11 March 2009)
Subject: Prince Charles was accused yesterday of using “quackery” to exploit gullible people after his Duchy Originals label launched a controversial detox tincture.
Quote: Andrew Baker, chief executive of Duchy Originals… said: “Duchy Herbals Detox Tincture is traded as a food supplement and in accordance with all of the relevant sections of both UK and European food laws. It is a natural aid to digestion and supports the body’s natural elimination processes. It is not – and has never been described as – a medicine, remedy or cure for any disease.”
Prof Ernst said: …“Products like this are a dangerous waste of money. Charles is exploiting gullible people during hard times. It’s outright quackery.” The academic, who has been a professor at Exeter for 15 years, labelled the Prince’s firm “Dodgy Originals”.
My comment (score): The subject matter was relevant, in my view. The article and my comments are both correct. (10)
6. Title (date of publication): Homeopathy: A ‘cure’ that is all in the mind? (11 February 2010)
Subject: Imagine if an electronics store publicly admitted that an entire range of the products it sold didn’t work. It wasn’t that the DVD players were not very good quality, it simply didn’t have any evidence that they played DVDs at all.
Quote: A report published yesterday by the House of Commons Science and Technology Committee said the products were no more effective than a dummy pill and recommended the NHS stop funding them. Back in October last year Paul Bennett, the professional standards director of Boots, appeared in front of the Committee’s inquiry into alternative medicine. When asked if he believed that homeopathic products worked he said: “There is certainly a consumer demand for these products. I have no evidence to suggest they are efficacious.”
Scientists say there is no evidence water has such a memory or that homeopathy works at all beyond a basic placebo effect. “The principles are simply implausible,” says Professor Edzard Ernst, Professor of Complementary Medicine at the Peninsular Medical School in Exeter. “It might be OK that the principle is implausible if the method still worked but rigorous clinical trials have demonstrated that the method doesn’t work. On both levels the result is negative.”
My comment (score): The subject matter was relevant. The article seems correct but my comment seems a bit confusing. (8)
7. Title (date of publication): Acupuncture ‘a waste of time’ for couples trying for a baby (10 March 2010)
Subject: Couples who have acupuncture to boost their chances of becoming parents are wasting their time and money, experts said yesterday.
Quote: New guidelines from the British Fertility Society, which represents fertility clinics, said there was “no evidence” that either acupuncture or traditional Chinese herbal remedies could improve the success rate of In-Vitro Fertilisation.
Edzard Ernst, professor of complementary medicine at the Peninsula Medical School, based at the universities of Exeter and Plymouth, said: “This is a long-overdue clarification. Infertile women have been misled for some time now to think that traditional Chinese medicine can help them getting pregnant. This analysis shows two things very clearly: The totality of the acupuncture trials does not support this notion, and for Chinese herbs, we have no evidence at all. This will help infertile women not to waste their money.”
My comment (score): The subject matter was relevant. The article seems correct and my comment is true. (10)
8. Title (date of publication): Prince Charles’s charity in £150 000 fraud quiz (4 April 2010)
Subject: One of Prince Charles’s charities is being investigated by police amid allegations of a £150,000 fraud.
Quote: The Prince’s Foundation for Integrated Health … which campaigns for the wider use of complementary therapies, has failed to file its annual return. According to the Charity Commission website it is 154 days overdue. A spokesman for the foundation said: “Due to staff and structural changes, there was a delay in preparing the 2008 accounts. While getting these accounts ready for filing, our auditors Kingston Smith questioned some of the transactions. A t their recommendation a complaint has been made to the police. ” … Dr Michael Dixon, medical director for the foundation, said: “We should not abandon patients we cannot help with conventional scientific medicine. If homeopathy is getting results for those patients then of course we should continue to use it.”
The complaint also claimed the foundation’s trustees allowed staff to pursue a “vendetta” against a prominent critic , Edzard Ernst, professor of complementary medicine at Exeter University. Republic accused the foundation of being partly responsible for the imminent closure of Professor Ernst’s department after he publicly attacked its draft guide to complementary medicines as “outrageous and deeply flawed”.
My comment (score): The subject matter was relevant. The article seems correct albeit slightly confusing (the ‘vendetta’ is not really relevant here) the quotes are somewhat beside the point; mine seems copied from elsewhere. (7)
9. Title (date of publication): Prince Charles’s charity amid £300k fraud inquiry (30 April 2010)
Subject: PRINCE Charles’s homeopathy charity has been shut down amid a Scotland Yard investigation into a £300,000 fraud.
Quote: The 49-year-old man was arrested on Monday with a 54-year-old woman, both on suspicion of the same offences, after an investigation into £300,000 of unaccounted funds in the charity’s books.
… while the foundation has enjoyed successes, sometimes working with the Prince’s Duchy Originals company to produce alternative health care products, it has also become embroiled in a series of controversies. Critics have accused it of promoting “unscientific” approaches to health care. In February, MPs on the Commons Science and Technology Committee called for an end to homeopathy treatment on the NHS, arguing there was no evidence to support its effectiveness. Edzard Ernst, professor of complementary medicine at Exeter University, last year described a detox tincture made by Duchy Originals as “outright quackery” and regulators ordered the firm to withdraw misleading advertising claims about the effectiveness of two natural remedies.
My comment (score): The subject matter was relevant. The article seems correct and my comment seems copied from elsewhere and is beside the point. (8)
10. Title (date of publication): ‘Snake oil seller’ Prince Charles cost me my job, claims professor (26 July 2011)
Subject: A university professor, who labelled Prince Charles and other supporters of complementary medicine as “snake-oil salesmen”, last night accused the heir to the throne of costing him his job.
Quote: Edzard Ernst, a consistent critic of Prince Charles and his Duchy Originals food company, is stepping down from his post at Exeter University as Britain’s only professor of complementary medicine after a long-running dispute with the Prince about the merits of alternative therapies. He said: “Almost directly, Prince Charles has managed to interfere in my professional life and almost managed to close my unit.” He blamed Charles, a prominent advocate of alternative therapies such as acupuncture, herbal remedies and homeopathy, for undermining him and leading his bosses to lose faith in him.
A spokeswoman for Charles claimed last night that the Prince was unaware that his private secretary had complained about the professor. She declined to respond to the description of her boss as a “snake-oil salesman”.
My comment (score): The subject matter was relevant. The article seems correct and my comment is true. (10)
11. Title (date of publication): Do detox diets work? (10 January 2012)
Subject: Most of us overdo it during the festive season. No wonder January is the most popular month for detox diets which typically involve drinking pints of water each day, eating a very restricted diet and taking particular supplements.
Quote: The theory is toxins from unhealthy types of food and drink build up in the body and can lead to health problems. Purging these toxins is meant to leave you feeling full of energy and thinner.
The principle of detox goes back to medieval times but it is anti-science, agrees Professor Edzard Ernst, Britain’s first professor of complementary medicine, who works at Peninsula College of Medicine & Dentistry in Exeter. “You can’t overindulge on food and drink, then wave some magic wand,” he says. “The only thing that detox removes is money from your wallet.”
My comment (score): The subject matter was relevant. The article seems correct and my comment is true. (10)
12. Title (date of publication): Kevin Sorbo: Three strokes left me fighting for my life (28 February 2012)
Subject: Becoming a key speaker at a medical conference may seem an unlikely part for a Hollywood tough guy. Nevertheless that’s the role Hercules star Kevin Sorbo took after breaking his silence over three life-threatening strokes.
Quote: He made an appointment with his chiropractor. “I had been seeing this guy for eight years and he never cracked my neck,” recalls Kevin. “He knew I didn’t like it.” So he was surprised when the therapist did crack his neck. When he asked him why, the chiropractor responded by saying “I felt you needed it”. Irritated, the star paid his bill and started driving back to the home of his girlfriend, now wife, Sam. “I heard two very loud pops in the back of my head and my vision went crazy. I felt like I was falling backwards and I couldn’t stop. It was like that feeling you get when you stand up too quickly and get dizzy but multiplied by 10,” he says. Kevin managed to drive to Sam’s apartment and despite hearing two more “pops” went on to appear on a TV chat show after his agent insisted he could not pull out at the last minute. “I don’t remember what we discussed. I was on auto-pilot. The entire world was spinning, my head was throbbing. It was the best acting of my life, acting as though I was healthy.”
Whether or not the cracking technique is dangerous is a controversial issue. A study by Professor Edzard Ernst, director of complementary medicine at the UK’s Peninsula Medical School says: “Numerous deaths have occurred after chiropractic manipulations.” He thinks the risks of this treatment by far outweigh its benefit and adds: “In my view a chiropractor should not go near the neck.”
However Haymo Thiel, vice-principal of the Anglo-European College of Chiropractic, says: “There is risk in anything. It would be foolish to say not. But there is a difference between coincidence of timing and causation.”
My comment (score): Even though this is merely a case report, the subject matter seems relevant. The article seems correct and my comments are true. The Thiel comment at the end might serve as a nice example of false balance. (8)
13. Title (date of publication): Menopause: Natural remedies vs HRT (29 January 2013)
Subject: Are natural remedies best for the menopause, or is HRT still the strongest defence against its many unpleasant symptoms?
Quote: Since two major studies called hormone replacement therapy into question a decade ago – raising fears of breast cancer, stroke and heart disease – women confronting the menopause have faced a confusing choice.
“Few of the herbal remedies have been properly studied,” says Edzard Ernst, professor of complementary medicine at the university of Exeter. “Some promising evidence has emerged for black cohosh and red clover, but even these are not as strongly beneficial as HRT.”
My comment (score): The subject matter was relevant. The article seems correct and my comment is true. (10)
14. Title (date of publication): How safe is our herbal medicine? (19 March 2013)
Subject: For many of us hoping to take care of our aches and pains, boost our immune system or improve our mood, herbal remedies are often the first resort. Seen as a healthier and more natural option than conventional medication few of us stop to ask how safe these supplements actually are.
Quote: High street health chain Holland & Barrett is the most recent to fall foul of these rules. In January it was ordered to recall a blend of black cohosh and agnus castus called Flash Fighters which it was selling as a food supplement. A spokesman for the chain confirmed: “The MHRA stated the product’s name implied it could be used to treat ‘hot flushes’.” He added that the store is undergoing the process of having Flash Fighters reclassified under the Traditional Herbal Medicine Registration Scheme (THR).
Professor Edzard Ernst, world’s first professor of complementary medicine, warns: “The notion that natural equals safe can be dangerously misleading.”
My comment (score): The subject matter was relevant. The article seems correct and my comment is true. (10)
15. Title (date of publication): Prince Charles SLAMMED as ‘immoral’ for peddling ‘rubbish’ alternative medicines (18 January 2018)
Subject: Charles is under fire from a renowned scientist who accuses him of being an “immoral snake oil salesman” for promoting alternative medicines in a shocking new book that lambasts the future monarch.
Quote: Professor Edzard Ernst, who previously accused Charles of “selling snake oil”, has now hit out with a new book called “More Harm than Good?” He scalds Charles for being a vocal supporter of homeopathy, lobbying health ministers to set up a register of holistic practitioners and making impassioned speeches at the World Health Assembly and British Medical Association. The authors of the book, Professor Ernst and Dr Kevin Smith, of Abertay University in Dundee, said alternative medicines are “immoral”. Professor Ernst said: “You can’t have alternative medicine just because Prince Charles likes it, because that is not in the best interest of the patients.
My comment (score): The basis for the article was a presentation of a new book at the ‘Science Media Centre’. The book merely mentioned Charles merely in passing. The article and our comments seem correct, however, they were not to focus of our presentation. (7)
16. Title (date of publication): Weight loss pills: Are they actually effective in helping you lose weight? (10 September 2018)
Subject: Weight loss pills claiming to help you lose weight, are widely advertised. But do they actually live up to their claims; are they effective in helping you to lose weight or are they simply a con?
Quote: More than one-third of adults are overweight in England alone, with nearly one-quarter obese, and growing numbers of people are turning to weight loss pills and products as a means to shed excess weight. Many weight loss pills claim to contain herbs or natural substances that speed up metabolism or make you feel full up to discourage you from eating. But according to the NHS, there is little evidence that some products sold by reputable retailers and over the internet actually work, and could even be packed with harmful substances. Even products marketed as ‘guaranteed, clinically-proven and 100 per cent natural’ come with no guarantees, the NHS warned.
Some manufacturers of weight loss products also only focus on positive trials, failing to mention the negative or failed trials. “Manufacturers cherry-pick and only ever mention the positive trials,” said academic physician and researcher Edzard Ernst. “They then also fail to mention the mostly poor quality of their studies. Desperate people are being misled to buy unproven treatments at considerable expense.”
My comment (score): The subject matter was relevant. The article seems correct and my comment is true. (10)
17. Title (date of publication): Prince Charles under fire for becoming patron of 175-year-old homeopathy group (26 June 2019)
Subject: The Prince of Wales has been criticised after being made a patron of a 175-year-old homeopathy group, which supports medical professionals with alternative treatments.
Quote: Charles has long advocated homeopathic medicine, which is seen as an alternative to regular chemical-based treatments. Homeopathy attempts to treat some conditions, including headaches and colds, so the body will get better by itself. But after Charles was accused of being an “immoral snake oil salesman” by a medical professor in 2017, it seems more are lining up to take aim at the future monarch for further endorsement of alternative medicine.
Professor Edzard Ernst, who made the initial criticisms of Charles last year, told the Guardian: “In view of Charles’s long love affair with homeopathy, this news is unsurprising. The question is whether this will change anything about the sharp decline homeopathy has taken in this and several other countries, and whether it will alter the verdicts of dozens of independent organisations which recently have certified it to be a pure placebo therapy.”
My comment (score): The subject matter was relevant. The article seems correct my quotes are borrowed from elsewhere. (7)
18. Title (date of publication): China sparks fresh coronavirus fears by turning to traditional medicine to fight virus (29 June 2020)
Subject: Chinese government papers have revealed that a shocking majority of the country’s cases have been treated with traditional medicine.
Quote: Coronavirus currently has very little universally approved and clinically proven treatments, but scientists have made some discoveries into potentially effective drugs.
Edzard Ernst, a retired UK-based researcher of complementary medicines, said that there is no science behind the recommendation to support it’s usage. He said to Nature: “For TCM there is no good evidence and therefore its use is not just unjustified, but dangerous.”
My comment (score): The subject matter was relevant. The article seems correct my quotes are borrowed from elsewhere. (7)
19. Title (date of publication): Prince Charles fury: Scientist’s shock claim royal ‘treated him like dirt’ exposed (1 July 2020)
Subject: Prince Charles is known to be enthusiastic about alternative medicines and therapies. Yet, Professor Edzard Ernst, who has several times criticised the royal for his influence in the world of pseudo-medicine, once claimed that the prince “silenced” and treated him “like dirt”, a shocking unearthed report revealed.
Quote:
Prince Charles for decades has welcomed alternative medicines and therapies to apparently “cure” his ailments. One of the pseudo-sciences most popular with the prince appears to be homeopathy. Homeopathy is the largely discredited practice of treating illness with diluted substances to trigger the body’s own healing mechanisms.
In 2015, Professor Edzard Ernst, claimed he had been “treated like dirt” as a result of Charles trying to “silence” him. Prof Ernst is a staunch critic of using alternative medicines such as homeopathy as a direct means of treatment. He instead champions complementary medicine – the process of using alternative medicines to help alleviate the negative aspects of standard medicines – having held the first complementary medicine post in the world at the University of Exeter. His unscrupulous and rigorous application of evidence-based science and outspoken views found him at loggerheads with Charles.
My comment (score): The subject matter was only marginally relevant. I am pretty sure that I never said Charles treated me like dirt. I did say, however, that my university did treat me like dirt when dealing with the complaint from Charles’s first private secretary, Sir Michael Peat. My comments are borrowed from elsewhere. (4)
20. Title (date of publication): Prince Charles’ ‘plot’ with Andy Burnham for UK healthcare unveiled: ‘He was open to it’ (23 October 2020)
Subject: Prince Charles was once in agreement with Andy Burnham on the future direction of the UK’s healthcare, letters have revealed.
Quote: …this is not the first time Mr Burnham has been caught up in a divisive matter over healthcare. In 2009, the Greater Manchester Mayor was the Health Secretary under then Prime Minister Gordon Brown and was found to be corresponding with the Prince of Wales about the UK healthcare system. Charles has a reputation for being a “meddling” royal, particularly after his so-called ‘black spider memos’ to Government ministers were published in 2015.
Professor of complementary medicine Edzard Ernst told The Guardian in 2015: “The letters demonstrate yet again that Prince Charles relentlessly meddles in UK health politics and thus disrespects his constitutional role. “His arguments in favour of CAM [complementary and alternative medicine] and in particular homeopathy, show a devastating lack of knowledge and understanding; they are ill-informed, invalid and embarrassingly naive – but at the same time they are remarkably persistent.”
My comment (score): The subject matter was relevant. The article seems confusing my quotes are borrowed from elsewhere. (7)
21. Title (date of publication): Meghan Markle warning: Charles’ business blunder exposed amid new career move (17 December 2020)
Subject: Meghan Marle has just moved into the business sector after investing in a start-up – but she should be careful to avoid Prince Charles’ previous industry error which triggered a public outcry.
Quote: The Duchess of Sussex has ventured into the investment sector this week. It was announced that she has invested in Clevr Blends, a California-based sustainable start-up which sells four flavours of instant oat milk lattes. The company says its produce is sustainable, ethically sourced and healthy with organic ingredients, while its shipping materials are 100 percent recyclable.
However, Meghan’s father-in-law was accused of exploiting the public when Britain was still recovering from the recession with his Duchy Originals line. The UK’s first professor of complementary medicine, Edzard Ernst, dubbed the Duchy Originals detox tincture — which was being sold on the market at the time — “outright quackery”. The product, called Duchy Herbals’ Detox Tincture, was advertised as a “natural aid to digestion and supports the body’s elimination processes” and a “food supplement to help eliminate toxins and aid digestion”. The artichoke and dandelion mix cost £10 for a 50ml bottle.
My comment (score): The subject matter seems fairly irrelevant and far-fetched. My quotes belong to a different story. (2)
22. Title (date of publication): Prince Charles rejected by experts before Gwyneth Paltrow’s long Covid row: ‘Witchcraft’ (25 February 2021)
Subject: Prince Charles was rejected by scientists for his views on “witchcraft” alternative medicine well before Gwyneth Paltrow became embroiled in a row over her unapproved treatments for long Covid.
Quote: Gwyneth Paltrow has been urged to stop spreading misinformation by the medical director of NHS England after she suggested on her blog Goop that long Covid could be treated with various alternative medicines. The Hollywood star described how she herself had caught coronavirus and had since suffered with “long-tail fatigue and brain fog”. However, she claimed to have successfully treated it with “intuitive fasting”, herbal cocktails and regular visits to an “infrared sauna”.
The Prince of Wales has been specifically called out for advocating the controversial treatments, too. He was branded an “immoral snake oil salesman” by renowned scientist Professor Edzard Ernst in his book ‘More Harm Than Good?’ Prof Ernst founded the department of Complementary Medicine at the University of Exeter, became the world’s first academic on the subject and has founded two medical journals. Over the years, he has published a lot of critical research exposing methods that lack documentation of efficacy. The expert lambasted Charles for lobbying health ministers to set up a register of holistic practitioners and making impassioned speeches at the World Health Assembly and British Medical Association. He said: “You can’t have alternative medicine just because Prince Charles likes it, because that is not in the best interest of the patients. “The quality of the research is not just bad, but dismal. It ignores harms. There is a whole shelf of rubbish being sold and that is simply unethical.” His co-author, Dr Kevin Smith ‒ a senior lecturer at Abertay University specialising in Complementary and Alternative Medicine and genetics ‒ agreed that these alternative medicines are “immoral”. He added: “We certainly are very worried about the future King being a proponent.
My comment (score): The subject matter seems fairly irrelevant and far-fetched. My quotes belong to a different story. (2)
________________________________________
When I set out doing this analysis, I expected to find rather poor reporting by the DE. Yet, I was pleasantly surprised. Quite a lot of it is good. A few things did nevertheless occur to me:
- I find it remarkable how often Prince Charles is the focus of these stories. Occasionally, my various disputes with Charles were ‘pulled in’ even though they do not really fit into the context of the article.
- It is noticeable, I think, that the quality of the reporting deteriorated quite dramatically over time.
- The DE repeatedly borrows quotes from other publications and even from different stories altogether. This seems to me to be lazy and rather poor journalism.
My point is that there is really no need for lazy or poor journalism on SCAM. Journalists should do their work properly; they can always reach me via the contact option of this blog (I invariably reply swiftly). I feel they owe it to their readers to do at least this minimal and quick amount of effort.
According to his own website , Andreas Kalcker is a biophysical researcher of German origin who has lived most of his life in Spain and for many years has been living in Switzerland where he has investigated and registered several international patents that deal with the therapeutic use of chlorine dioxide for both hypoxia and for inflammation, infection, sepsis and Sars -Cov 2 -Coronavirus.
, Andreas Kalcker is a biophysical researcher of German origin who has lived most of his life in Spain and for many years has been living in Switzerland where he has investigated and registered several international patents that deal with the therapeutic use of chlorine dioxide for both hypoxia and for inflammation, infection, sepsis and Sars -Cov 2 -Coronavirus.
In recent years, he seems to have been particularly active as a snake oil salesman in South America. Argentinian authorities have now charged Andreas Kalcker for promoting toxic bleach (MMS) as a “miracle” medical treatment. Kalcker, alongside several Argentinian nationals, is accused of playing a key role in promoting chlorine dioxide in the country as a cure for various illnesses, including COVID-19, in conferences, books, and on social media.
The charges follow a seven-month-long investigation by the Unidad Fiscal para la Investigación de Delitos contra el Medio Ambiente (UFIMA), which investigates medical crimes in Argentina. The investigation was launched after the August 2020 death of a five-year-old boy in Neuquen, western Argentina, of multiple organ failure consistent with chlorine dioxide poisoning. The child’s parents believed, on the basis of misinformation spread by Kalcker and others, that the substance had the power to ward off COVID-19. An Argentinian judicial source said that Kalcker has been charged with the illegal practice of the medical profession and selling fake medicines. If found guilty of causing a child’s death, Kalcker could serve a prison sentence of up to 25 years.
Apart from Kalcker, four other persons were accused of being responsible for the distribution of chlorine dioxide in Argentina. The Argentine nationals had advertised and sold the substances via the internet – apparently in Kalcker’s name. According to the prosecution, “this distribution would have led to the messages about the ‘improvements’ resulting from the consumption of a substance with serious health consequences, which can even lead to death, being circulated with greater vigor.” The lawyer who started the ball rolling through his complaint is convinced that the parents of the deceased child believed that chlorine dioxide could protect their child from COVID-19 because of the misinformation spread by Kalcker.
Chlorine dioxide is a type of industrial bleaching agent commonly used to treat wood products. Public health authorities around the world have issued warnings about taking the substance, with the US Food and Drugs Administration warning that it can be fatal if taken in large doses. In recent years, a movement originating in a fake Florida “church” has promoted the substance it calls “Miracle Mineral Solution” (MMS), or “Chlorine Dioxide Solution” (CDS), as a cure for a range of illnesses and conditions.
MMS, or the Miracle Mineral Supplement, is a beverage product designed by former aerospace engineer, Jim Humble, who has tested his MMS protocol in Malawi and other parts of Africa. Initially used to treat malaria, the manufacturer claims field-tested success in treating and reversing the effects of AIDS, malaria, hepatitis, herpes, tuberculosis, most cancers, and a host of other diseases.
MMS has been promoted with the help of celebrities and VIPs, including Donald Trump. One of the many websites that advertise MMS states the following about it:
Master Mineral Solution, MMS or WPS Solution – Why has this Product Become so Popular?
Chlorine dioxide is a powerful anti microbial compound that has a long history of use – mostly known for its ability to sanitize drinking water (the last 60 years being the primary chemical used in municipal water supplies). The reason being is that it works, & works well. There are very few pathogens out there in water anywhere in the world that cannot be made potable with the use of this potent little molecule.
I think it goes without saying that MMS has not been shown to be effective against any condition while being very harmful when taken orally by humans.