big pharma
It has been reported that HomeoCare Laboratories Inc. is recalling two batches of Homeopathic StellaLife Oral Care Products citing microbial contamination. The recall involves Homeopathic Stella Life Vega Oral Care Spray Unflavored and Advanced Formula Peppermint Oral Care Rinse manufactured in 2024, which are marketed to promote oral health, hydrate oral cavities and support healthy gums. The recall is to be performed at the consumer level.
StellaLife VEGA Oral Care, Spray Unflavored comes with NDC 69685-121-01, lot no. 2552 and expiration date of 02-2026. StellaLife Advanced Formula Peppermint VEGA Oral Care Rinse comes with NDC 69685-143-16, lot no. 2550, and expiration date of 02- 2026.
The affected products were manufactured at HomeoCare Laboratories, shipped nationwide, and distributed through various dental practices. As per the FDA, higher than acceptable levels of TAMC was found in the Advanced Formula Peppermint Vega Oral Care Rinse, while Bacillus sp was found in the StellaLife Vega Oral Spray, Unflavored. Bacillus is a common species found in the environment and are generally non-pathogenic, while patients with oral disease, undergoing dental surgical procedures or with compromised immune systems hold potential risks. In the immunocompromised population, the impacted product may cause severe or life-threatening adverse events due to the introduction of bacteria to the disrupted oral mucosa, possibly leading to bacteremia and sepsis. However, the manufacturer of homeopathic products has not received any reports of adverse events related to these two recalled products so far.
Dental practices and consumers, who have the recalled products, are urged to return the impacted products to HomeoCare Laboratories or to the place of purchase or discard them. The company said it is implementing enhanced quality control measures to prevent recurrence.
On the manufacturer’s website, we find the following:
Homeopathy is a safe, gentle, and natural system of healing that works with your body to relieve symptoms, restore itself, and improve your overall health. It is safe to use and has none of the side effects of many traditional medications, because it is made from the natural substances and is FDA regulated. Homeopathic medicines – known as “remedies” – are made from natural sources (e.g., plants, minerals), and are environmentally friendly and cruelty free.
Homeopathic remedies when used as directed, are completely safe for everyone. They are given in such small doses that they don’t cause side effects.* Homeopathy is not a general or “umbrella” term that describes a variety of different natural therapies. Although homeopathic remedies are derived from natural substances, homeopathy should not be confused with herbal medicine, Chinese medicine, or other types of natural medicines. It is its own, unique therapeutic system.
The FDA’s present policy does not require homeopathic medicines to go through the FDA approval process. The homeopathic ingredients monographed in the Homeopathic Pharmacopoeia of the United States have been reviewed for homeopathic efficacy, toxicology, adverse effects and clinical use. The historical safety record with the use of homeopathic drugs, some for close to 200 years. The FDA drug monitoring process does not reveal any significant instances of problems with homeopathic drug products, thus establishing a positive safety profile.
Homeopathy’s Basic Principle: The Law of Similars It is accepted knowledge that every plant, mineral, and chemical can cause in overdose its own unique set of physical, emotional, and mental symptoms. It also is readily acknowledged that individuals, when ill, have their own idiosyncratic physical, emotional, and mental symptom patterns, even when people have the same disease. Homeopathic medicine is a natural pharmaceutical science in which a practitioner seeks to find a substance which would cause in overdose similar symptoms to those a sick person is experiencing. When the match is made, that substance then is given in very small, safe doses, often with dramatic effects.
Homeopaths define the underlying principle for this matching process as the “law of similars.” The “law” is not unknown to conventional medicine. Immunizations are based on the principle of similars. No less a person as Dr. Emil Adolph Von Behring, the “father of immunology,” directly pointed to the origins of immunizations when he asserted, “By what technical term could we more appropriately speak of this influence than by Hahnemann’s word “homeopathy.”
Homeopathy is a natural form of medicine used by over 200 million people worldwide. The holistic nature of homeopathy means each person is treated as a unique individual and their body, mind, spirit and emotions are all considered in the management and prevention of disease. Taking all these factors into account a homeopath will select the most appropriate medicine based on the individual’s specific symptoms and personal level of health to stimulate their own healing ability.
Homeopathic medicines are safe to use as they rarely cause side-effects. This means when used appropriately under the guidance of a qualified homeopath they can be taken by people of all ages*.
* Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated. Individual results may vary.
__________________________
I feel like congratulating the manufacturer: not only have they managed to produce normally harmless products in such a way that they are dangerous, but also they are promoting a plethora of untruth and misleading statements about homeopathy. A most remarkable effort!
I usually take ‘market reports’ with a pinch of salt. Having said that, this document makes some rather interesting predictions:
The size of the market for so-called alternative medicine (SCAM) is projected to expand from USD 147.7 billion in 2023 to approximately USD 1489.4 billion by the year 2033. This projection indicates a remarkable Compound Annual Growth Rate (CAGR) of 26% over the forecast period.
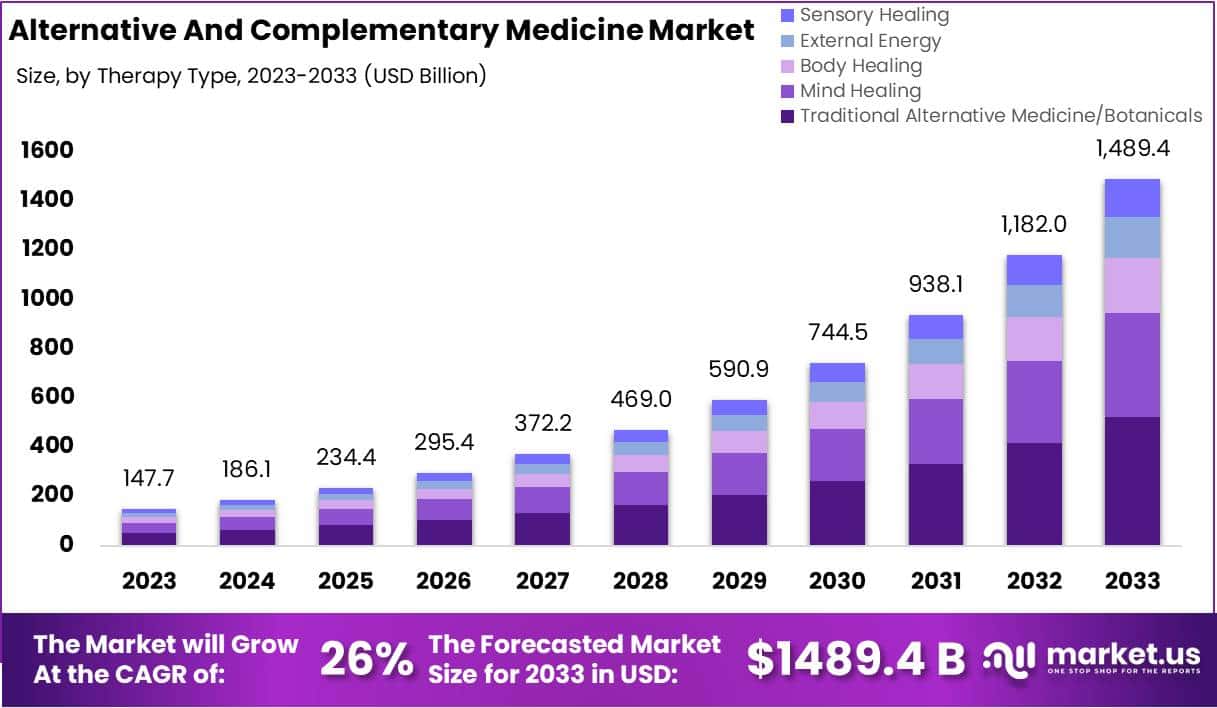
The market for SCAM is experiencing significant growth, fueled by increasing consumer interest in natural and holistic health solutions. This trend reflects a broader shift in societal attitudes towards health and wellness, emphasizing preventive care and natural health practices.
The market’s dynamics are influenced by various factors, including consumer preferences, regulatory standards, and evolving perceptions of health and wellness. As the popularity of these alternative therapies grows, it is crucial for individuals to consult with healthcare professionals to ensure that these non-conventional approaches are safely and effectively incorporated into their overall health regimen. The increasing acceptance of SCAM underscores a collective move towards more personalized and holistic healthcare solutions, resonating with today’s health-conscious consumers.
In 2023, Traditional Alternative Medicine/Botanicals led the market, capturing a 35.2% share, which reflects a strong consumer inclination towards these treatments. Dietary Supplements were prominent in the market, securing a 25.1% share in 2023, which underscores the high consumer demand for nutritional aids. Direct Sales were the most favored distribution channel, accounting for 43.2% of the market share in 2023, which indicates their significant impact on guiding consumer purchases. Pain Management was the predominant application area, holding a 24.9% market share in 2023, propelled by the growing acknowledgment of non-pharmacological treatment options. Adults represented a substantial portion of the market, making up 62.33% in 2023, signifying a marked preference for SCAM therapies within this age group. Europe stood out as the market leader, claiming a 42.6% share in 2023, a position supported by widespread acceptance, governmental backing, and an increasing elderly population. The regions of North America and Asia-Pacific are highlighted as areas with potential, signaling opportunities for market expansion beyond the European stronghold in the upcoming years.
Leading Market Players Are:
- Columbia Nutritional
- Nordic Nutraceuticals
- Ramamani Iyengar Memorial Yoga Institute
- The Healing Company Ltd.
- John Schumacher Unity Woods Yoga Centre
- Sheng Chang Pharmaceutical Company
- Pure encapsulations LLC.
- Herb Pharm
- AYUSH Ayurvedic Pte Ltd.
Recent developments:
- In December 2023, Adoratherapy launched the Alkemie Chakra Healing Line, an aromatherapy range aimed at harmonizing the seven chakras.
- Coworth Park introduced the Hebridean Sound Treatment in October 2023, merging traditional Hebridean sounds with guided meditation to offer a novel, restorative wellness experience.
- The World Health Organization released draft guidelines in September 2023 for the safe, effective application of traditional medicines.
- Telehealth services, expanding significantly in August 2023, have broadened the reach of SCAM, enhancing patient access to these treatments.
Proponents of so-called alternative medicine (SCAM) are often – as we had many opportunities to observe here on this blog – not impressed with the safety and efficacy of COVID vaccinations. This is despite the fact that several studies have demonstrated the huge number of lives saved by them, both at national and multi-country level in the earlier stages of the pandemic. I wonder whether the doubters will be convinced by new evidence.
This analysis estimates how many lives were directly saved by vaccinating adults against COVID in the Region, from December 2020 through March 2023.
The researchers estimated the number of lives directly saved by age-group, vaccine dose and circulating Variant of Concern (VOC) period, both regionally and nationally, using weekly data on COVID-19 mortality and COVID-19 vaccine uptake reported by 34 European areas and territories (CAT), and vaccine effectiveness (VE) data from the literature. They calculated the percentage reduction in the number of expected and reported deaths.
The authors found that vaccines reduced deaths by 57% overall (CAT range: 15% to 75%), representing ∼1.4 million lives saved in those aged ≥25 years (range: 0.7 million to 2.6 million): 96% of lives saved were aged ≥60 years and 52% were aged ≥80 years; first boosters saved 51%, and 67% were saved during the Omicron period.
The authors concluded that over nearly 2.5 years, most lives saved by COVID-19 vaccination were in older adults by first booster dose and during the Omicron period, reinforcing the importance of up-to-date vaccination among these most at-risk individuals. Further modelling work should evaluate indirect effects of vaccination and public health and social measures.
The authors feel that their results reinforce the importance of up-to-date COVID-19 vaccination, particularly among older age-groups. Communication campaigns supporting COVID-19 vaccination should stress the value of COVID-19 vaccination in saving lives to ensure vulnerable groups are up-to-date with vaccination ahead of periods of potential increased transmission.
Those SCAM proponents who are not convinced of the merits of COVID and other vaccinations will undoubtedly claim that this new analysis was biased and thus unreliable. Therefore, it seems worth stating that this work was supported by a US Centers for Disease Control cooperative agreement, who had no role in data analysis or interpretation. The authors affiliated with the World Health Organization (WHO) are alone responsible for the views expressed in this publication and they do not necessarily represent the decisions or policies of the WHO.
2003 has been marked by many terrifying things, but perhaps the most surprising of the 2023 horrors was … eye drops. ArsTechnica reports that the seemingly innocuous teeny squeeze bottle made for alarming headlines numerous times during our current revolution around the sun, with lengthy lists of recalls, startling factory inspections, and ghastly reports of people developing near-untreatable bacterial infections, losing their eyes and vision, and dying.
Recapping this unexpected threat to health, the Food and Drug Administration released an advisory titled “What You Should Know about Eye Drops” with this stark pronouncement: No one should ever use any homeopathic ophthalmic products, and every single such product should be pulled off the market.
The point is unexpected, given that none of the high-profile infections and recalls this year involved homeopathic products. But, it should be welcomed by any advocates of evidence-based medicine.
In the US, these products are marketed as legitimate treatments and sold alongside evidence-based treatments (though consumer advocates are trying to change that). The reason this is allowed for now is because of a regulatory quirk: Based on the 1938 Food, Drug, and Cosmetic Act, homeopathic products are generally considered exempt from pre-market FDA safety and efficacy reviews as long as the active ingredient in the product is included in the Homeopathic Pharmacopoeia.
In recent years, the FDA and the Federal Trade Commission have cracked down on homeopathic products, though. And it seems from today’s advisory that the FDA is not holding back on homeopathic products for the eyes. The regulator notes that any products meant for the eye “pose a heightened risk of harm” because the eyes are an immune-privileged site in the body. That is, innate immune responses are restrained in the eye to prevent damaging inflammation, which could threaten vision. “Any drug used in the eyes must be sterile to reduce the risk of infection,” the FDA said.
But whether or not homeopathic eye drops are labeled as sterile doesn’t seem to matter to the FDA. The regulator cautions:
“Do not use ophthalmic products that are labeled as homeopathic, as these products should not be marketed.”
SAY NO MORE!
I just found this on ‘X’ (formerly Twitter):
We’re delighted to announce the launch of the #BeyondPills All Parliamentary Group in Westminster. Chaired by Danny Kruger MP and co-chaired by Lord Crisp, this new body aims to tackle #overreliance on pills, reducing the number of unnecessary and inappropriate prescriptions.
It turns out that I did not study the website of College of Quackery and Integrated Health as regualarly as I should have. Because there, the launch had been announced some time ago under the title ‘Beyond Pills All Party Parliamentary Group (APPG) launches to stop over-prescribing‘:
Now, in December 2023, we have an exciting development to report: the launch of the Beyond Pills All Party Parliamentary Group (APPG), in which the Beyond Pills Campaign joins forces with the former APPG for Prescribed Drug Dependence. We’re delighted to announce that the APPG’s former Chair, Danny Kruger MP, joins the new Beyond Pills APPG as Chair.
Danny Kruger said of the launch: ‘There is a natural synergy with our objectives and the APPG for Prescribed Drug Dependence is a great supporter of social prescribing, which we feel can make a valuable contribution to addressing this public and personal health crisis, both in terms of helping to prevent overprescribing and also to treating people who are suffering from the debilitating symptoms of dependence.’
The Beyond Pills Campaign now becomes a founder member of the Beyond Pills Alliance (BPA), alongside the Council for Evidence-based Psychiatry (CEP-UK). Setting up the BPA will, in the near future, give us the opportunity to invite other organisations with a similar goal of reducing overreliance on pills to join the Alliance.
The Beyond Pills APPG has the following Mission and Objectives:
MISSION: To move UK healthcare beyond an over-reliance on pills by combining social prescribing, lifestyle medicine, psychosocial interventions and safe deprescribing. As well as reducing unnecessary and inappropriate prescribing, this integrated approach will improve outcomes and reduce health inequalities.
Elsewhere, Dr. Michael Dixon, who seems to be in charge of the ‘beyond pills’ activities, explained: ‘The Beyond Pills All Party Parliamentary Group heralds a sea change in public perception and medical practice from “a pill for every ill” to recognising that there is so much that we can do for ourselves which will not only help us to heal but also stop us getting ill in the first place.’
Sea change?
Really?
This made me think – and eventually, I respond by writing this short ‘open letter’ to the group:
Dear ‘BEYOND PILLS All Parliamentary Group‘
please let me begin by stating that I am all in favour of reducing over-prescribing. Who isn’t? The clue is in the name ‘over– prescribing’! Yet, at the same time, I would like to alert you to the fact that your group’s name ‘beyond pills‘ is of questionable merit.
It implies that conventional medicine consits only or predominantly of prescribing pills. My own career as a clinician – long ago now – was in physical medicine and rehabilitation, a discipline that certainly does not rely on pills. Many other areas of healthcare also do not exclusively rely on pills; take surgery or psychosomatic medicine, for instance. As for the rest of the physicians, they will, no doubt, have learnt in medical school that over-prescribing is wrong, dangerous, and not evidence-based.
By putting ‘beyond pills’ on your banner, you either disclose your ignorance of the facts, or you deliberately undermine trust in conventional medicine. Some less benevolent than I might even get the impression that you employ the ‘strawman fallacy‘ in order to push a hidden agenda.
I hope these lines might motivate you to reconsider and alter the irresponsible name of your initiative – how about ‘evidence-based medicine’?
Sincerely
Edzard Ernst
PS
In case anyone wants to use my ‘open letter’ on other sites or publication, I herewith grant permission to reproduce it.
The British doctor and outspoken anti-vaxer Aseem Malhotra has featured several times on this blog, e.g.:
- UK Cardiologist Dr. Aseem Malhotra receives a well-deserved award
- Dr Aseem Malhotra and Dr Steven James: candour and complacency
Now, there has been a potentially important new development in his story. The Good Law Project recently announced the following:
During the pandemic, we depended on doctors telling us how we could protect ourselves and our loved ones. We trusted their advice would be based on the most reliable and up-to-date research.
But when the British cardiologist Dr Aseem Malhotra went on television, or posted to his hundreds of thousands of followers on social media, he repeatedly claimed the vaccine was ineffective and posed a greater threat than Covid, causing “horrific unprecedented harms including sudden cardiac death” – suggestions refuted by medical experts and branded false by factcheckers.
The General Medical Council is responsible for regulating doctors in the UK and investigating those whose conduct falls short of the required standards. Despite the clear risk to public health of vaccine misinformation, it has so far refused to launch an investigation into Malhotra’s public pronouncements, originally saying that they “don’t consider that the comments or posts made by the doctor call his fitness to practice into question…” and subsequently upholding that decision after a number of doctors challenged it.
Good Law Project is supporting a doctor who is taking the regulator to the High Court over their failure to investigate whether Malhotra has breached standards. The judicial review has now been given permission to proceed by the High Court, which held that it raises an “issue of general public importance” as to how the GMC exercises its functions.
According to the claimant, Dr Matt Kneale, medical professionals “should not be using their professional status to promote harmful misinformation”.
“When doctors repeatedly say things that are incorrect, misleading and put people’s health at risk – for example by encouraging them to refuse a vaccine – the GMC must hold them to account,” Kneale said.
For the Good Law Project Executive Director, Jo Maugham, the regulator’s failure to investigate doctors spreading misinformation forms part of a wider pattern.
“What we have learned from both the pandemic inquiry and the calamitous economic consequences of Brexit,” Maugham explained, “is quite how serious are the consequences of deciding, as Michael Gove did, that we have ‘had enough of experts’.”
The council may prefer to avoid becoming embroiled in a controversy over free speech, he continued, but “its primary obligation is to protect the public – and it’s really hard to see how its stance delivers on that objective.”
Dr Malhotra is far from the only proponent of vaccine misinformation in the UK. Open Democracy revealed that anti-lockdown MPs, including Tufton Street’s Steve Baker, took large donations from a secretive group called The Recovery Alliance, which has been linked with a fake grassroots organisation that campaigned against the vaccine.
We’re working to stop misinformation from going unchallenged, and to make sure that regulators like the General Medical Council hold dangerous doctors who make unfounded claims accountable.
By helping to fund this case, you’ll be fighting for trust in the medical profession and to make sure public safety is doctors’ first priority. Any support you can give will help us make positive change.
____________________
The ‘Good Law Project’?
Who are they?
Good Law Project is a not for profit campaign organisation that uses the law for a better world. We know that the law, in the right hands, can be a fair and decent force for good. It is a practical tool for positive change and can make amazing things happen. We are proud to be primarily funded by members of the public, which keeps us fiercely independent. We want to inspire hope in difficult times by showing that you can make a difference, with the backing of good law. Our mission is to use the law to hold power to account, protect the environment, and ensure no one is left behind. You can learn more about our organisation and achievements in 2022-23 in our annual report.
You might even decide to support this splendid organization!
I hope you do.
Guest post by Ken McLeod
Readers will recall that Barbara O’Neill is an Australian health crank, completely unqualified in anything, who is subject of a Permanent Prohibition Order issued by the New South Wales Health Care Complaints Commission, (HCCC),[1] preventing her from engaging in any health-related activity, including ‘health education,’ in Australia. The NSW Public Health Act 2010 provides that it is an offence for a person to provide ‘health education’ in contravention of a prohibition order, with a fine of $60,500 AUD ($38,151 USD, 36251 Euros) for an individual or imprisonment for 3 years, or both, or $121,000 AUD for a corporation.
For jurisdictional reasons that Order does not apply outside Australia and for several years she been touring the world giving health education lectures. The latest was a lecture tour of Ireland.[2] Despite the thorough debunking of her fruitloop beliefs by the HCCC,[3] she has maintained them and continues to give the ‘health education’ that was so dangerous that it led to the Prohibition Order in Australia.
Her Irish ‘health education’ lectures were live-streamed to people in Australia who paid the 20 Euro fee, and one was recorded by us.[4]
A transcript was made and is available online.[5] Her statements were analysed and some comments are made as follows. Alas, we didn’t have time to take a deep dive of her lecture to find the best references, but the following shows that an amateur with limited time and resources can prove that she does not know what she is talking about and that her advice is dangerous, even life-threatening.
It is up to the health regulators and immigration authorities in each country to act on her activities there, but so far none outside Australia have done so.
So a quick analysis of her ‘lecture’ in Dublin on 27 September 2023 shows that O’Neill has learned nothing from her experience with the HCCC. Some comments:
1. O’Neill and her husband, after the Prohibition Order was issued, changed the name of their facility from ‘Misty Mountain Health Retreat’ to ‘Misty Mountain Lifestyle Retreat’ to avoid the jurisdiction of the HCCC. However on four occasions in her lecture O’Neill referred to it as a ‘health retreat.’ 00:07:23 , 00:15:48, 01:30:04, 01:40:16.
2. At 00:12:53 O’Neill claims that the Amish don’t get autism. That is false, as explained by AP Factcheck. [6]
3. At 00:12:54 O’Neill claims that the Amish, ‘They don’t vaccinate their Children. Did you know that they don’t vaccinate their Children and yet they don’t get autism Very rare. Maybe 1%. And often that’s because of chemical exposure. There is always a reason. So why are vaccinations causing autism? Well, it’s neurotoxins, the neurotoxins. ‘
False; Amish do vaccinate their children. [7] However, studies have documented cases of autism, diabetes and cancer among the Amish, albeit at lower rates in some cases than the broader population and for reasons that are unrelated to their vaccination status. These reasons include the cultural norms and customs that may be playing a role in the reporting style of caregivers. [8] O’Neill is engaging in cherry-picking on a grand scale here.
4. At 00:13:37 O’Neill claims that ‘there are still two more neurotoxins’ (In vaccines.) Because children are still autistic. There’s formaldehyde, and there is aluminium, both neurotoxins.’
This is scaremongering disinformation. The CDC says ‘Formaldehyde is diluted during the vaccine manufacturing process, but residual quantities of formaldehyde may be found in some current vaccines. The amount of formaldehyde present in some vaccines is so small compared to the concentration that occurs naturally in the body that it does not pose a safety concern.’ As for aluminium, the CDC says ‘Ingredients like aluminum salt help boost the body’s response to the vaccine.’ The CDC says that both are safe. [9]
5. At 00:15:01 O’Neill claims ‘did you know that the milk in the supermarket if you give that to a newborn baby cow, that cow will die?’
I can find no reference supporting that and I suggest that it is pure fantasy.
6. At 00:18:29 O’Neill claims that ‘parents discover that they put their trust in the princes and vaccinated their child. Now their child has epilepsy. Now their child has autism.’
This is misleading panic-mongering that is a misrepresentation of the science. The Royal Australian College of General Practitioners says ‘Seizures and status epilepticus can occur within 14 days following administration of inactivated and live-attenuated vaccines. These vaccine-proximate seizures can undermine parental confidence in vaccine safety and affect further vaccination decisions. Vaccine-proximate status epilepticus (VP-SE) is uncommon but may be the first manifestation of genetic developmental epileptic encephalopathies, including Dravet syndrome.’ So ‘epilepsy’ may be first encountered [10] following vaccination but the root cause is genetic.
7. At 00:20:27 O’Neill says that she would like to suggest that no child would be vaccinated, because the fact is, our body was designed to heal itself.
This is pure crazy antivax propaganda, unsupported by the facts.
8. At 00:22:01 O’Neill claims ‘skin cancer has only been around in about the last 80 years, and you know what they’re finding today? That vitamin D deficiency is a big contributing back factor to skin cancer’.
The first claim is false; the science shows that skin cancers have been around ‘since the beginning of time.’ [11]
As for the second claim, the research published at the US National Library of Medicine shows that O’Neill’s advice is dangerous. ‘It is, therefore, preferable and safer to obtain adequate levels of vitamin D through diet than through sun exposure. In fact, it is currently accepted that dietary and supplemental vitamin D is functionally identical to that produced after UV exposure, being more reliable and quantifiable (the risks of keeping high levels of vitamin D have not been extensively studied) source of this vitamin.’ And ‘Neither natural nor artificial sun exposure should be encouraged as the main source of vitamin D.’ [12]
9. At 00:23:18 O’Neill disputes claims that ‘cholesterol causes heart disease. Well, it’s been going for 40 years now, and it still hasn’t proven that. But you know what? It has proven that people with high cholesterol levels don’t get Alzheimer’s.’
O’Neill’s first claim points to the conflicting research as revealed by the Cochrane Collaboration. [13] As for her second claim, the research does not justify her claim that it is ’proven.’ The evidence is conflicting and as the Alzheimer’s Society of the UK say, ‘More research is needed to better understand this relationship and what it can tell us.’ [14] O’Neill’s conviction is not based on evidence.
10. At 00:34:41 O’Neill said that at Dublin airport ‘about 10 days ago,’ she was approached by a man who asked ‘Are you the Australian doctor? And I smiled.’
O’Neill did not correct him and allowed him to be duped into believing she is a real doctor. Despite having no qualifications in anything O’Neill has used the honorific title ‘Dr’ many times in social media,[15] so it is no surprise that he assumed she was a doctor. I can’t help but be confused by her use of the ‘Dr.’ Throughout her lectures she denigrates real doctors, and then tries to boost her credibility by adopting the title.
11. At 00:35:21 she claimed that with ‘epigenetics, you can actually turn your genes on or off.’…. ‘So Michael effectively turned those genes off with castor oil. Castor is very effective for for cataracts. Put it in your eye, one lady said. Is it safe? Does anyone ever ask that of the doctor? Is that drug safe? Then the people have been putting cholesterol in their eyes for centuries. It’s safe.’
Bollocks! As Consumer Lab says ‘Although eye drops containing castor oil may help improve symptoms of dry eye and blepharitis, there is currently no compelling evidence that applying castor oil to the eye can diminish cataracts.’ [16] And there is no evidence that Michael turned the genes off.
12. At 00:40:08 she refers to a woman who recently had a stroke. She says
‘… because she had a stroke, she was put on the protocol she was on put on statins. Cholesterol lowering medication with clear arteries. How much sense does that make? You don’t have. You don’t have to be a rocket scientist to work this out. Trust in your gut feeling trust in this incredible body that God has given you. Her blood was no longer thick. Her arteries are open now. And so she came to our retreat and I said, Well, I can’t tell you what to do. And I have no authority over your medication. Only you, and go. You and your doctor do. But this is what I would do. I would stop the blood thinning medication immediately because that aspirin causes brain bleeds, eye bleeds and stomach bleeds. Got that? And I would stop the statin drugs because that the side effect of statin drugs is Alzheimer’s dementia, uh, memory loss, muscle wasting. And they’ve just added another one, which is breast cancer, because all our sex hormones are made from cholesterols.’
O’Neill told a woman who had suffered a stroke to stop taking her life-saving medication! These medications are prescribed by highly qualified medical specialists based on the research. As the UK Stroke Association says, ‘Blood-thinning medications reduce your risk of stroke by helping to prevent blood clots from forming. You might be prescribed them after a transient ischaemic attack (TIA) or a stroke caused by a blockage (an ischaemic stroke, or clot).’[17] It is clear that O’Neill, who has no qualifications in anything, does not know what she is talking about.
As for her claim that the side effects of statins is breast cancer, the research shows the opposite. ‘While statins do not affect the incidence of most cancers, they do exert significant benefits on recurrence and survival in many cancer types, including breast cancer.’ [18]
13. At 42:48 O’Neill claims ‘If you are on cholesterol lowering medication and many have been deceived….’ As above, it is O’Neill who is doing the deceiving.
14. At 45:09 O’Neill claims that ‘If you stop your cholesterol lowering medication, there will be a side effect. Your memory will return. Your muscles will get stronger. Any little appearances of Alzheimer’s will start to ease.’
As above, the available research does not show that.
15. At 48:57 O’Neill claims ‘Why did they put fluoride in water? The claim was to harden the teeth. Has it hardened the teeth? Not at all. Has it reduced tooth decay? Not at all.’ And ‘But that fluoride is very hard on the kidneys, very hard on the liver.’
The research here is overwhelming; as the CDC says: ‘The CDC named community water fluoridation one of 10 great public health achievements of the 20th century.
‘Many research studies have proven the safety and benefits of fluoridated water. For 75 years people in the United States have been drinking water with added fluoride and enjoying the benefits of better dental health.
‘Drinking fluoridated water keeps teeth strong and reduces cavities (also called tooth decay) by about 25% in children and adults.’
As for O’Neill’s claim that fluoride is very hard on the kidneys, very hard on the liver,’ the research is inconclusive, and in fact the reverse may be true. Research shows ‘Fluoride exposure may contribute to complex changes in kidney and liver related parameters among U.S. adolescents. As the study is cross-sectional, reverse causality cannot be ruled out; therefore, altered kidney and/or liver function may impact bodily fluoride absorption and metabolic processes.’ So the science does not support O’Neill’s certainty.
16. At 48:57 O’Neill claims that ‘all body symptoms and body diseases and shows how dehydrating has a huge factor.’ O’Neill gives no evidence to support that huge claim.
17. At 01:00:20 O’Neill claims that a woman told her ‘I had the vaccine. Now I’ve got clots. Barbara, I had the vaccine. I can’t. I cannot even remember all the diseases that are arising. Have you noticed? And so many people were blackmailed into that vaccine.’ And ‘Is that (COVID19) a crisis? it’s not a crisis at all. And yet we’re seeing so many problems arising.’
O’Neill is dreadfully wrong here. COVID 19 was a crisis. How else would we describe a pandemic that is known to have killed at least 6,961,014 deaths, as reported to the WHO? [19] And what are the problems that we are seeing arising? Outside her imagination, that is.
18. At 01:00:20 O’Neill claims that ‘one man said, Show me the safety studies. They gave him three pages of blank paper. No safety studies, no safety studies at all.’ (On vaccines). And ‘drugs never cure disease.’ And a few lines later, again, ‘Drugs never cure disease.’
The allegation that ‘They (doctors) gave him three pages of blank paper’, is just so deranged. No doctor would do that because there are thousands of studies of vaccine safety.
O’Neill’s claim that there are no safety studies on vaccines is hopelessly wrong and dishonest. It’s one of the many anti-vax lies circulating on the internet, so beloved by the gullible. As the Australian Dept of Health and Aged Care say, ‘Research and testing is an essential part of developing safe and effective vaccines. In Australia, every vaccine must pass strict safety testing before the Therapeutic Goods Administration (TGA) will register it for use. Before vaccines become available to the public, they are tested on thousands of people who take part in large clinical trials.’ [20] It took me a few seconds on the internet to find an interesting research paper on HPV vaccines, including a section on safety. [21] O’Neill could do that so the inevitable conclusion is that she set out to deceive. As for ‘drugs never cure disease,’ that is so bizarre, so whacky, so deluded, that it almost not worth challenging. But I will anyway; medical professionals have seen drugs work billions of times, and I can testify that I was saved from a life-threatening illness due to cephalexin.
19. At 01:10:49 O’Neill claims ‘some (medications) can be stopped immediately, like your statin drugs and your blood thinners. Yeah, what do you take instead of statin drugs? Well, there’s no need, because cholesterol is not a problem.’
O’Neill’s advice here is life-threatening rubbish. As the Mayo Clinic says ‘Abruptly stopping an anticoagulant can increase your risk of a stroke.’ [22] As for her advice on cholesterol, see above.
20. At 01:15:39 O’Neill claims that there was ‘No diabetes on the planet til sugar was well established.’ And lack of nose-breathing causes ‘Chronic fatigue syndrome. There’s one cause; it’s lack of oxygen at the cellular level.’
Humans have gathered sugar since we first became homo sapiens and diabetes has always been a problem for us and other animals.
As for her claim that lack of nose-breathing causes ‘Chronic fatigue syndrome;’ the Mayo Clinic says ‘The cause of ME/CFS is unknown, although there are many theories. Experts believe it might be triggered by a combination of factors.’ They go on to list many possible causes but lack of nose-breathing is not one of them.[23]
21. At 01:26:08 O’Neill claims that a researcher ‘…. could turn cancer cells on and off by the amount of animal, pro and animal protein that he was giving’ and liver cancer could be prevented by ‘a simple diet and cancer weights were very low low compared to the city again, with that high meat diet….’ There is some truth in this, but it does not justify O’Neill’s other advice to avoid prescribed medications.
22. At 01:49:26 O’Neill claims ‘if someone has a rash and they put cortisone on it, what happens to the rash? It’s gone, but But it comes back in about another week. Is that right? Twice as bad.’ And ‘No drug can heal cancer. The body and the body alone when it’s given the right conditions can cause cancer to be conquered in the body.’ And ‘A fever is nothing to fear.’
O’Neill’s claim that ‘No drug can heal cancer’ is demonstrably wrong. Life expectancy following cancer treatment has improved vastly over the decades, largely due to better detection and prescribed medications. As the US National Cancer Institute (NCI) estimates, ‘due to improved detection and treatment, deaths have dropped 41 percent from 1989 to 2018, according to the ACS.’ [24]
As for O’Neill’s claim that ‘a fever is nothing to fear,’ the Victorian Dept of Health says ‘High fever (about 41.5°C or more) is extremely dangerous and could trigger convulsions.’ [25]
23. At 01:53:47 O’Neill claims that drug therapy is not working.
What does O’Neill mean by that? Does she mean that prescribed medication does not work? If she is repeating her earlier claim that ‘drugs never cure disease?’ I repeat my earlier rebuttal. That is so bizarre, so whacky, so deluded, that it almost not worth challenging. But I will anyway; medical professionals have seen drugs work billions of times, and I can testify that I was saved from a life-threatening illness due to cephalexin.
I’ll finish the analysis here because you have suffered enough.
Readers everywhere now have rock-solid evidence that should be presented to their national health regulators, showing that O’Neill, as the HCCC put it, ‘poses a risk to the health and safety of members of the public’ and therefore ‘should be permanently prohibited from providing any health services, whether in a paid or voluntary capacity.’ And you have rock-solid evidence that should be presented to venue managers who have allowed O’Neill to present life-threatening ‘education’ to the public on their premises, asking them to cancel the booking. It’s not hard; it was done in Ireland by members of the public. That led to cancellation of the booking, and a rush by O’Neill’s supporters to find a new venue.
References
1 https://www.hccc.nsw.gov.au/decisions-orders/public-statements-and-warnings/public-statement-and-statement-of-decision-in-relation-to-in-relation-to-mrs-barbara-o-neill
2 https://www.independent.ie/irish-news/controversial-wellness-coach-barbara-oneill-set-to-host-talk-in-ireland-this-month/a1781099169.html
3 https://www.hccc.nsw.gov.au/ArticleDocuments/216/Statement%20of%20Decision%20-%20Mrs%20Barbara%20ONeill.pdf.aspx
4 The video is available at https://rumble.com/v3lt611-barbara-oneill-positive-life-event-27th-september.html and a backup is available at https://www.dropbox.com/scl/fi/vqe9plhgjijunvl22kvb6/Barbara-ONeill-Positive-Life-Event-27th-September.mp4?rlkey=1kjyi9jdl8kfdp8kcdf1p4xba&dl=0
5 https://www.dropbox.com/scl/fi/csl95hg7gomr318nygotx/TRANSCRIPT-BARBARA-O-NEILL-POSITIVE-LIFE-EVENT-DUBLIN-27-SEPT-2023.pdf?rlkey=z2d5uh59fwagzdfdk30hvpauy&dl=0
6 https://apnews.com/article/fact-check-amish-covid-vaccines-cancer-diabetes-autism-356029928165
7 https://apnews.com/article/fact-check-amish-covid-vaccines-cancer-diabetes-autism-356029928165
8https://www.researchgate.net/publication/268144514_Prevalence_Rates_of_Autism_Spectrum_Disorders_Among_the_Old_Order_Amish
9 https://www.cdc.gov/vaccines/vac-gen/additives.htm
10 https://www1.racgp.org.au/ajgp/2020/october/seizures-following-vaccination-in-children
11 https://www.usatoday.com/story/news/factcheck/2023/08/03/false-claim-skin-cancer-has-only-been-around-for-60-years-fact-check/70515019007/
12 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8709188/
13 https://s4be.cochrane.org/blog/2018/07/02/cholesterol-and-heart-disease-whats-the-evidence/
14 https://www.alzheimers.org.uk/about-dementia/risk-factors-and-prevention/cholesterol-and-dementia
15 https://www.facebook.com/people/Dr-Barbara-ONeill/100093111507726/
16 https://www.consumerlab.com/answers/castor-oil-eye-drops-for-cataracts/castor-oil-cataracts/
17 https://www.stroke.org.uk/resources/blood-thinning-medication-and-stroke
18 https://breast-cancer-research.biomedcentral.com/articles/10.1186/s13058-018-1066-z#author-information
19 https://covid19.who.int/
20 https://www.health.gov.au/are-vaccines-safe
21 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7565290/
22 https://connect.mayoclinic.org/blog/take-charge-healthy-aging/newsfeed-post/know-the-warning-signs-of-blood-thinner-complications/
23 https://www.mayoclinic.org/diseases-conditions/chronic-fatigue-syndrome/symptoms-causes/syc-20360490
24 https://www.healthline.com/health/breast-cancer/survival-facts-statistics#breast-cancer-stages
25 https://www.betterhealth.vic.gov.au/health/conditionsandtreatments/fever#bhc-content
Vaccine hesitancy has become a threat to public health, especially as it is a phenomenon that has also been observed among healthcare professionals.
In this study, an international team of researchers analyzed the relationship between endorsement of so-called alternative medicine (SCAM) and vaccination attitudes and behaviors among healthcare professionals, using a cross-sectional sample of physicians with vaccination responsibilities from four European countries:
- Germany,
- Finland,
- Portugal,
- France.
In total the sample amounted to 2,787 physicians.
The results suggest that, in all the participating countries, SCAM endorsement is associated with lower frequency of vaccine recommendation, lower self-vaccination rates, and being more open to patients delaying vaccination, with these relationships being mediated by distrust in vaccines. A latent profile analysis revealed that a profile characterized by higher-than-average SCAM endorsement and lower-than-average confidence and recommendation of vaccines occurs, to some degree, among 19% of the total sample, although these percentages varied from one country to another:
- 24% in Germany,
- 18% in France,
- 10% in Finland,
- 6% in Portugal.
These results constitute a call to consider health care professionals’ attitudes toward SCAM as a factor that could hinder the implementation of immunization campaigns.
The authors also point out that the link between SCAM endorsement and negative attitudes toward vaccines has been documented in previous research among the general public. A systematic review, which categorized arguments against vaccines retrieved from peer-reviewed articles and debunking texts published by international fact checking agencies, identified a category of arguments largely based on alternative health beliefs related to SCAM. This category was the third most common in the scientific and fact-checking literature. Furthermore, in a British study, anti-vaccination arguments related to SCAM were also among the most endorsed arguments by individuals. These results suggest that SCAM beliefs play an important role in individuals’ justification of their hesitant attitudes toward vaccines for both adults and children. Analyses of samples from the Australian, Finnish, American, and Spanish general populations found that positive attitudes toward SCAM were related to negative attitudes toward vaccines. In a recent large-scale study in 18 European countries, parental consultation with homeopaths was associated with higher vaccine hesitancy than consultation with pediatricians or nurses. Moreover, a systematic review found that SCAM use tended to be positively associated with lower childhood immunization. Similar findings were reported also from the US and Australia.
There are several potential causes for the observed relationship between vaccine hesitancy and SCAM. Since SCAM use occurs more frequently at the poles of the disease spectrum (i.e., in cases of minor or life-threatening illness), SCAM use has been identified as a marker of both misperception of risk and frustration with regular healthcare (e.g., negative prognosis or lack of remission of symptoms). Accordingly, SCAM-related health conceptions could be motivating healthcare practitioners (HCPs) to be more reluctant to recommend and receive vaccinations both for illnesses that are perceived as minor and in cases of severe clinical pictures. There are also reasons related to the potential alignment between SCAM and the ideology or worldview of the HCP, such as their distrust in “Big Pharma” or a general disregard for scientific knowledge. Along the same lines, it has been shown that the main reasons for their preference for SCAM included a greater affinity between SCAM, their do-it-yourself approach to health care, and their sympathy for natural and allegedly harm-free products in contrast to medications marketed by pharmaceutical companies, which were perceived as ineffective, “toxic” and “adulterating.”
Besides these implicit reasons, some SCAM traditions are theoretically incompatible with vaccination and portrayed as a valid, or even superior, alternative to scientific knowledge. A quantitative study found that pro-SCAM and anti-vaccination attitudes both reflect beliefs contrary to basic scientific knowledge, such as “an imbalance between energy currents lies behind many illnesses” and “an illness should be treated with a medicine that has properties similar to those of the illness.” An example of these SCAM-related beliefs that contradict the theoretical basis of vaccinations is “homeopathic immunization” through so-called “nosodes” – orally administered extreme dilutions of infectious agents. Similarly, Rudolf Steiner and Ryke Geerd Hamer, promoters of anthroposophic medicine and ‘German New Medicine’, respectively, have sown doubts about vaccinations based on their conceptions of the etiology and treatment of diseases. Consequently, strong science denial and vaccine hesitancy can be found within these communities, and outbreaks of vaccine-preventable diseases, such as measles and whooping cough, have been reported in educational centers linked to anthroposophy.
PS
This project has received funding from the European Union’s Horizon 2020 research and innovation programme.
The US ‘Public Citizen‘ is an American non-profit, progressive consumer rights advocacy group, and think tank based in Washington, D.C. They recently published an article entitled “FDA Guidance on Homeopathic Drugs: An Ongoing Public Health Failure“. Here are a few excerpts:
In December 2022, the U.S. Food and Drug Administration (FDA) issued new guidance on homeopathic drug products. The guidance states that the agency now “intends to apply a risk-based enforcement approach to the manufacturing, distribution and marketing of homeopathic drug products.”
Under this new risk-based approach, the agency plans to target its enforcement actions against homeopathic drug products marketed without FDA approval that fall within the following limited categories:
- products with reports of injury that, after evaluation, raise potential safety concerns
- products containing or purportedly containing ingredients associated with potentially significant safety concerns (for example, infectious agents or controlled substances)
- products that are not administered orally or topically (for example, injectable drug products and ophthalmic drug products)
- products intended to be used to prevent or treat serious or life-threatening diseases
- products for vulnerable populations, such as immunocompromised individuals, infants and the elderly
- products with significant quality issues (for example, products that are contaminated with foreign materials or objectionable microorganisms)
But this new FDA guidance fails to adequately address the public health threat posed by the agency’s decades-long permissive approach to these illegal drug products.
Under FDA regulations, prescription and over-the-counter (OTC) homeopathic products are considered drugs and are supposed to be subject to the same review and approval requirements as all other prescription and OTC medications. However, under a flawed enforcement policy issued in 1988, the FDA has allowed these drug products to be marketed in the U.S. without agency review or approval. Thus, all products labeled as homeopathic are being marketed without the FDA having evaluated their safety, effectiveness or quality…
… there is no plausible physiologic or medical basis to support the theory underlying homeopathy, nor is there evidence from well-designed, rigorous clinical trials showing that homeopathic drugs are safe and effective.
The FDA should declare unequivocally that all unapproved homeopathic drug products are illegal and direct all manufacturers to immediately remove such products from the market. In the meantime, as we have recommended for many years, consumers should not use homeopathic products. At best, the products are a waste of money, given the lack of any evidence that they are effective. At worst, they could cause serious harm because of the lack of FDA oversight to ensure safety.
_____________________
I fully agree with these sentiments. The harm caused by homeopathy is considerable and multi-facetted. Many previous posts have discudded these problems, e.g.:
- Nine cases of severe homeopathy-induced liver injuries
- Another death by homeopathy
- HOMEOPATHY – “It is not just irresponsible, it’s downright dangerous.”
- Adverse effects of homeopathy and aggravations at NAFKAM
- Homeopathy: it’s time to stop the double standards
- Homeopathy can cause serious harm – and finally, the NHS England has realised it
- Vidatox, homeopathy’s answer to cancer or outright fraud?
- Another child has died because of homeopathy
- Doctor homeopaths violate fundamental rules of ethics when practising homeopathy
- ‘Best homeopathy doctor in Delhi’ offers treatment for HIV/AIDS
- DIY-Homeopathy: how to kill your entire family
- The risks of homeopathy?
- The FDA has warned 4 manufacturers of unapproved injectable homeopathic drugs
- Is this the crown of the Corona-idiocy? Nosodes In Prevention And Management Of COVID -19
- The FDA has sent more warning letters to homeopathic manufacturers
- Walmart is being sued for selling homeopathic products
- Homoeopathic remedies may be safe, but do all homeopaths merit this attribute?
- Recommending homeoprophylaxis is unethical, irresponsible and possibly even criminal
- FDA: homeopathic teething remedies were toxic
- “Homeoprophylaxis, the homeopathic vaccine alternative, prevents disease through nosodes.”
- A truly dangerous homeopath
- The scandalous attitude of some homeopaths and their supporters towards immunisations
- Oh yes, let’s have homeopaths as primary care practitioners! But only in a parallel universe,please.
Having warned about the dangers of homeopathy for decades, I feel it is high time for regulators across the world to take appropriate action.
It has been reported, at the German Medical Congress (DÄT) a year ago, that it was decided to delete the additional title of homeopathy from the model further training regulations of the German Medical Association. And Federal Health Minister Karl Lauterbach (SPD) tweeted applause: “Homeopathy has no place in modern medicine.”
Now the ‘ Bundesverband der Pharmaziestudierenden in Deutschland’ (BPhD), the German Pharmacists Organization, even goes a few steps further. The position paper distinguishes between evidence-based medicine (EBM) and unproven therapeutic methods. According to the BPhD, these include homeopathy, but also anthroposophy, traditional Chinese medicine, and traditional medicines.
Among other things, the BPhD is disturbed by the way homeopathy presents itself as an alternative, because an alternative means “a choice between two equally suitable possibilities” to achieve a goal, and this is not the case. Compared to evidence-based medicine (EBM), homeopathy is a “constructed, illusory concept” and “the principles of homeopathic teachings and principles” are to be rejected as “unscientific”. According to the BPhD, a designation as “alternative” for advertising purposes should no longer be allowed.
They would also like to see a demarcation from naturopathy; the clear distinction between homeopathy and phytopharmacy has been lacking up to now. The advertising attribute “natural” should therefore also be banned in order to prevent equalization in advertising, the position paper states.
Like doctors, pharmacy students point to the lack of proof of efficacy beyond the placebo effect. According to the BPhD, the dogma WER HEILT HAT RECHT, “he who heals is right” would “disregard all processes that work towards healing and glorify the result”. The “gold standard” of EBM – randomized, double-blind studies with placebo control – should in future also have to be fulfilled by homeopathic medicines, experience reports are not sufficient, it continues.
Homeopathic medicines are only registered as medicinal products without indication, which requires neither proof of efficacy nor clinical studies. The BPhD, therefore, demands that a warning be placed on the preparations that they have “no proven efficacy beyond the placebo effect”. Up to now, without this warning, patients have been “deceived about the efficacy”, and there is an “urgent need for detailed public information and counseling on homeopathy since its unjustified reputation poses a danger of not seeking treatment”. The BPhD also demands that the status of homeopathic medicines is withdrawn and that the pharmacy obligation for the preparations is abolished…
“In the health professions, no trivialization of unproven therapeutic procedures should be tolerated, as inadequate counseling or ignorance poses a danger to patients,” the BPhD said.
_________________________
When I first read this article – I translated and shortened it for those who cannot read German- I was truly dazzled. These are the suggestions that I have been making for around 20 years now, not specifically for Germany but for pharmacists in general. For many years, the Germans seemed the least likely to agree with me. But now they seem to be ahead of everyone else in Europe!
How come?
I suspect and hope that our recent initiative might have something to do with it.
Let’s hope that the pharmacists of other countries follow the German example.
