aromatherapy
The London-based ‘International Federation of Aromatherapists‘ claims that:
medical research has now recognised that emotional support is vital in maintaining good mental health. Wellbeing needs to encourage pleasant emotions of contentment and calmness, enabling feelings of relaxation, inner peace and feelings of optimism.
The simple act of caring touch (massage) using essential oils is a highly effective way to target symptoms of stress and anxiety and the following are a list of some of the most effective:
• Clary sage (Salvia sclarea)
• Neroli (Citrus aurantium var amara flos)
• Lavender (Lavandula angustifolia)
• Roman Chamomile (Chamaemelum nobile)
• Rose (Rosa damascena)
• Ylang ylang (Cananga odorata)
• Sweet orange (Citrus sinensis)
• Mandarin (Citrus nobilis)
• Petitgrain (Citrus aurantium var amara fol)
• Geranium (Pelargonium graveolens)
• Frankincense (Boswellia sacra)
“Caring touch using essential oils is a highly effective way to target symptoms of stress and anxiety”? And what about the list of the “most effective oils”? I concucted a few ‘rough and ready’ Medline searches and fear that there are some corrections in order.
- Clary sage (Salvia sclarea): no sound evidence for the the above claims.
- Neroli (Citrus aurantium var amara flos): only very limited evidence for the above claims.
- Lavender (Lavandula angustifolia): limited evidence exists (e.g. here).
- Roman Chamomile (Chamaemelum nobile): limited evidence exists (e.g. here).
- Rose (Rosa damascena): limited evidence exists (e.g.here).
- Ylang ylang (Cananga odorata): limited evidence exists (e.g. here).
- Sweet orange (Citrus sinensis): very limited evidence exists (e.g. here).
- Mandarin (Citrus nobilis): the only study I found was negative.
- Petitgrain (Citrus aurantium var amara fol): no sound evidence for the the above claims.
- Geranium (Pelargonium graveolens): no sound evidence for the the above claims.
- Frankincense (Boswellia sacra): no sound evidence for the the above claims.
So, for once there actually is some (albeit not convincing) evidence which should be good news for fans of aromatherapy/essential oils.
Why then does the International Federation of Aromatherapists not just go with the existing evidence?
Why do they claim things that are not true?
Why do they bring aromatherapy in more than necessary disrepute?
But perhaps they can prove my review of the evidence to be incomplete and can add convincing trial data to it?
Watch this space.
As promised, here is my translation of the article published yesterday in ‘Le Figaro’ arguing in favour of integrating so-called alternative medicine (SCAM) into the French healthcare system [the numbers in square brackets were inserted by me and refer to my comments listed at the bottom].
So-called unconventional healthcare practices (osteopathy, naturopathy, acupuncture, homeopathy and hypnosis, according to the Ministry of Health) are a cause for concern for the health authorities and Miviludes, which in June 2023 set up a committee to support the supervision of unconventional healthcare practices, with the task of informing consumers, patients and professionals about their benefits and risks, both in the community and in hospitals. At the time, various reports, surveys and press articles highlighted the risks associated with NHPs, without pointing to their potential benefits [1] in many indications, provided they are properly supervised. There was panic about the “booming” use of these practices, the “explosion” of aberrations, and the “boost effect” of the pandemic [2].
But what are the real figures? Apart from osteopathy, we lack reliable data in France to confirm a sharp increase in the use of these practices [3]. In Switzerland, where it has been decided to integrate them into university hospitals and to regulate the status of practitioners who are not health professionals, the use of NHPs has increased very slightly [4]. With regard to health-related sectarian aberrations, referrals to Miviludes have been stable since 2017 (around 1,000 per year), but it should be pointed out that they are a poor indicator of the “risk” associated with NHPs (unlike reports). The obvious contrast between the figures and the press reports raises questions [5]. Are we witnessing a drift in communication about the risks of ‘alternative’ therapies? [6] Is this distortion of reality [7] necessary in order to justify altering the informed information and freedom of therapeutic choice of patients, which are ethical and democratic imperatives [8]?
It is the inappropriate use of certain NHPs that constitutes a risk, more than the NHPs themselves! [9] Patients who hope to cure their cancer with acupuncture alone and refuse anti-cancer treatments are clearly using it in a dangerous alternative way [10]. However, acupuncture used to relieve nausea caused by chemotherapy, as a complement to the latter, is recommended by the French Association for Supportive Care [11]. The press is full of the dangers of alternative uses, but they are rare: less than 5% of patients treated for cancer according to a European study [12]. This is still too many. Supervision would reduce this risk even further [13].
Talking about risky use is therefore more relevant than listing “illusory therapies”, vaguely defined as “not scientifically validated” and which are by their very nature “risky” [14]. What’s more, it suggests that conventional treatments are always validated and risk-free [15]. But this is not true! In France, iatrogenic drug use is estimated to cause over 200,000 hospital admissions and 10,000 deaths a year [16]. Yes, some self-medication with phytotherapy or aromatherapy does carry risks… just like any self-medication with conventional medicines [17]. Yes, acupuncture can cause deep organ damage, but these accidents occur in fewer than 5 out of every 100,000 patients [18]. Yes, cervical manipulations by osteopaths can cause serious or even fatal injuries, but these exceptional situations are caused by practitioners who do not comply with the decree governing their practice.[19] Yes, patients can be swindled by charlatans, but there are also therapeutic and financial abuses in conventional medicine, such as those reported in dental and ophthalmology centres. [20]
Are patients really that naive? No. 56% are aware that “natural” remedies can have harmful side-effects, and 70% know that there is a risk of sectarian aberrations or of patients being taken in by a sect [21]. In view of the strong demand from patients, we believe that guaranteeing safe access to certain NHPs is an integral part of their supervision, based on regulation of the training and status of practitioners who are not health professionals, transparent communication, appropriate research, the development of hospital services and outpatient networks of so-called “integrative” medicine combining conventional practices and NHPs, structured care pathways with qualified professionals, precise indications and a safe context for treatment.[22] This pragmatic approach to reducing risky drug use [17] has demonstrated its effectiveness in addictionology [23]. It should inspire decision-makers in the use of NHPs”.
- Reports about things going wrong usually do not include benefits. For instance, for a report about rail strikes it would be silly to include a paragraph on the benefits of rail transport. Moreover, it is possible that the benefits were not well documented or even non-existent.
- No, there was no panic but some well-deserved criticism and concern.
- Would it not be the task of practitioners to provide reliable data of their growth or decline?
- The situation in Switzerland is often depicted by enthusiasts as speaking in favour of SCAM; however, the reality is very different.
- Even if reports were exaggerated, the fact is that the SCAM community does as good as nothing to prevent abuse.
- For decades, these therapies were depicted as gentle and harmless (medicines douces!). As they can cause harm, it is high time that there is a shift in reporting and consumers are informed responsibly.
- What seems a ‘distortion of reality’ to enthusiasts might merely be a shift to responsible reporting akin to that in conventional medicine where emerging risks are taken seriously.
- Are you saying that informing consumers about risks is not an ethical imperative? I’d argue it is an imperative that outweighs all others.
- What if both the inappropriate and the appropriate use involve risks?
- Sadly, there are practitioners who advocate this type of usage.
- The recommendation might be outdated; current evidence is far less certain that this treatment might be effective (“the certainty of evidence was generally low or very low“)
- The dangers depend on a range of factors, not least the nature of the therapy; in case of spinal manipulation, for instance, about 50% of all patients suffer adverse effects which can be severe, even fatal.
- Do you have any evidence showing that supervision would reduce this risk, or is this statement based on wishful thinking?
- As my previous comments demonstrate, this statement is erroneous.
- No, it does not.
- Even if this figure is correct, we need to look at the risk/benefit balance. How many lives were saved by conventional medicine?
- Again: please look at the risk/benefit balance.
- How can you be confident about these figures in the absence of any post-marketing surveillance system? The answer is, you cannot!
- No, they occur even with well-trained practitioners who comply with all the rules and regulations that exist – spoiler: there hardly are any rules and regulations!
- Correct! But this is a fallacious argument that has nothing to do with SCAM. Please read up about the ‘tu quoque’ and the strawman’ fallacies.
- If true, that is good news. Yet, it is impossible to deny that thousands of websites try to convince the consumer that SCAM is gentle and safe.
- Strong demand is not a substitute for reliable evidence. In any case, you stated above that demand is not increasing, didn’t you?
- Effectiveness in addictionology? Do you have any evidence for this or is that statement also based on wishful thinking?
My conclusion after analysing this article in detail is that it is poorly argued, based on misunderstandings, errors, and wishful thinking. It cannot possibly convince rational thinkers that SCAM should be integrated into conventional healthcare.
PS
The list of signatories can be found in the original paper.
Aromatherapy is popular yet it has a problem: there is no indication for it. Yes, it can make you feel better but this is hardly a true medical indication. I know of many things that make me feel better, and I would not call them a THERAPY! But perhaps this new study from Iran offers a solution for the dilemna:
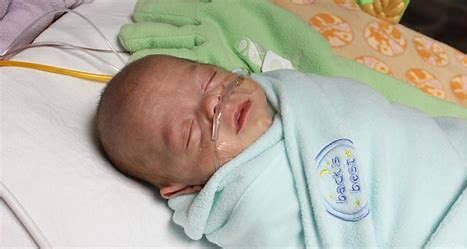
Sleep plays an essential role in infant development. This randomized clinical trial investigated the effect of aromatherapy with rose water on the deep sleep status of premature infants admitted to a neonatal intensive care unit (NICU).
The study was conducted on 64 infants hospitalized in NICUs. In the intervention group, two drops of rose water were poured on gas and placed next to the babies’ heads. The control group was treated in the same way except that distilled water was employed. The ALS scale was used to assess the sleep status.
Of the 66 infants in this study, 30 were female and 36 were male. The average gestational age of the infants was 32.5 ± 1.99 weeks. The results showed that the amount of deep sleep (type A and B) in the intervention group was significantly higher than the control group during and after the intervention (p=0.001).
The authors concluded that, considering the positive impact of rose water in improve of sleep quality in premature babies; it can be used to improve sleeping condition of infants in hospitals, along with main treatment.
The study has many flaws and it is badly written. Yet, I find it interesting. If its results can be confirmed with a more rigorous trial, aromatherapy might finally find a true medical purpose.
Every now and then, I like to look at what our good friend and SCAM entrepreneur Gwyneth Paltrow is offering via her extraordinary ripoff called GOOP. When I recently browsed through her goodies, I find lots of items that made me blush (common decency does not permit me to go into details here). But I also found something that I am sure many of us might need after the over-indulgence of recent weeks:Preview Changes (opens in a new tab)
“The Martini” Emotional Detox Bath Soak
The product is described as follows:
This body-and-spirit-centering bath soak, infused with Himalayan pink salt, helps take the edge off during turbulent times (or after a crazy day). Called “The Martini” after the traditional name for the last take of the day in filmmaking, the soak is made with pharmaceutical-grade Epsom salts, chia-seed oil, passionflower, valerian root, myrrh, Australian sandalwood, and wild-crafted frankincense.
Here at goop we believe in making every choice count, which is why we’ve always been outspoken about the toxic ingredients used in personal-care and beauty products (all are effectively unregulated in this country). We’re also passionate about the idea that beauty comes from the inside out. So we use clinically proven and best-in-class ingredients at active levels to create skin care, skin-boosting ingestibles, and body essentials that are luxurious, deliver high-performance results, and enliven the senses with exquisite textures and beautiful scents. We don’t rest until we think our products are perfect—safe enough and powerful enough for noticeable results. (All our products are formulated without parabens, petroleum, phthalates, SLS, SLES, PEGs, TEA, DEA, silicones, or artificial dyes or fragrances. And our formulas are not tested on animals.) We hope you love them as much as we do.
Yes, there is a whole world out there of which a retired chap like myself knows as good as nothing. And it has its very own terminology: 
- emotional detox
- body-and-spirit-centering
- pharmaceutical-grade Epsom salts
- wild-crafted
- clinically proven and best-in-class ingredients
- skin-boosting ingestibles
- body essentials
- high-performance results
By now, I am sure, you are dying to learn what the Emotional Detox Bath Soak contains:
Sodium Chloride, Magnesium Sulfate, Passiflora Incarnata Extract, Valeriana Officinalis Root Extract, Salvia Hispanica Seed Oil, Helianthus Annuus (Sunflower) Seed Oil, Rosmarinus Officinalis (Rosemary), Leaf Extract, Maltodextrin, Boswellia Carterii Oil, Commiphora Myrrha Oil, Fusanus Spicatus Wood Oil, Cyperus Scariosus (Nagarmotha) Oil, Vetiveria Zizanoides Root Oil, Simmondsia Chinensis (Jojoba) Seed Oil, Tocopherol.
Clinically proven, you ask?
Well, perhaps not in the sense that sad, retired academics tend to understand the term, but you have to realize, this is a different world where words have different meanings, the meaning entretreneurs want them to have. What is proven though is this: at $40 a tiny jar, the detox bath will eliminate some cash from your pocket – after all, that’s what detox is all about, isn’t it?
The Sunday Times reported yesterday reported that five NHS trusts currently offer moxibustion to women in childbirth for breech babies, i.e. babies presenting upside down. Moxibustion is a form of Traditional Chinese Medicine (TCM) where mugwort is burned close to acupuncture points. The idea is that this procedure would stimulate the acupuncture point similar to the more common way using needle insertion. The fifth toe is viewed as the best traditional acupuncture point for breech presentation, and the treatment is said to turn the baby in the uterus so that it can be delivered more easily.
At least four NHS trusts are offering acupuncture and reflexology with aromatherapy to help women with delayed pregnancies, while 15 NHS trusts offer hypnobirthing classes. Some women are asked to pay fees of up to £140 for it. These treatments are supposed to relax the mother in the hope that this will speed up the process of childbirth.
The Nice guidelines on maternity care say the NHS should not offer acupuncture, acupressure, or hypnosis unless specifically requested by women. The reason for the Nice warning is simple: there is no convincing evidence that these therapies are effective.
Campaigner Catherine Roy who compiled the list of treatments said: “To one degree or another, the Royal College of Midwives, the Care Quality Commission and parts of the NHS support these pseudoscientific treatments.
“They are seen as innocuous but they carry risks, can delay medical help and participate in an anti-medicalisation stance specific to ‘normal birth’ ideology and maternity care. Nice guidelines are clear that they should not be offered by clinicians for treatment. NHS England must ensure that pseudoscience and non-evidence based treatments are removed from NHS maternity care.”
Birte Harlev-Lam, executive director of the Royal College of Midwives (RCM), said: “We want every woman to have as positive an experience during pregnancy, labour, birth and the postnatal period as possible — and, most importantly, we want that experience to be safe. That is why we recommend all maternity services to follow Nice guidance and for midwives to practise in line with the code set out by the Nursing and Midwifery Council.”
A spokeswoman for Nice said it was reviewing its maternity guidelines. NHS national clinical director for maternity and women’s health, Dr Matthew Jolly, said: “All NHS services are expected to offer safe and personalised clinical care and local NHS areas should commission core maternity services using the latest NICE and clinical guidance. NHS trusts are under no obligation to provide complementary or alternative therapies on top of evidence-based clinical care, but where they do in response to the wishes of mothers it is vital that the highest standards of safety are maintained.”
On this blog, we have repeatedly discussed the strange love affair of midwives with so-called alternative medicine (SCAM), for instance, here. In 2012, we published a summary of 19 surveys on the subject. It showed that the prevalence of SCAM use varied but was often close to 100%. Much of it did not seem to be supported by strong evidence for efficacy. We concluded that most midwives seem to use SCAM. As not all SCAMs are without risks, the issue should be debated openly. Today, there is plenty more evidence to show that the advice of midwives regarding SCAM is not just not evidence-based but also often dangerous. This, of course, begs the question: when will the professional organizations of midwifery do something about it?
The tales of Kate Moss’s excesses are legendary. Sex and drugs and rock ‘n’ roll have always been an integral part of the supermodel’s life. Stories of wild behavior, random sexual encounters, and copious drug use seemed endless. Now, it seems she is adding a new element to her tumultuous career:
The supermodel is the latest in the long line of VIPs jumping on the quackery bandwagon by marketing her very own brand of over-priced nonsense. She was reported to have worked with Victoria Young, a homeopath and “spiritual guide”, on the products. There’s a Dawn Tea at £20 for 25 tea bags, “inspired by Kate’s English garden” – “With ingredients like hibiscus, rosemary, and nettle leaf, this first step of the Dawn Ritual gently energizes and strengthens the body”. There’s also a Dusk Tea.
There is also a 100ml bottle called Sacred Mist for £120. It is described as “a unique eau de parfum blended with essential oils for the body and surroundings.” There’s a 30ml bottle for £105 called Golden Nectar, which is pro-collagen. CBD oil drops to “holistically support body, mind, and soul”. A 50ml face cream for £95. A 100ml face cleanser for £52.
The website of Moss’ new enterprise claims that “COSMOSS draws on the extraordinary life experience of Kate Moss — someone whose career and image has touched on and influenced so many others and yet has taken her own, rich journey of transformation gradually and privately. COSMOSS is a celebration of every day exactly as it is, with all its imperfections. Each product has been meticulously crafted with wellbeing in mind, using potent, natural substances. Each ritual opens a door to balance, restoration, and love; each fragrance and infusion recentres and completes. COSMOSS is self-care created for life’s modern journeys to make them beautiful, mesmerising and magical.”
In a far cry from her past, Moss explained: “I’ve been meditating, doing yoga, just being much healthier. All the stuff that can make you feel more grounded and balanced.”
Personally, I am glad to hear that Kate is off cocaine and now into other, less harmful ‘natural substances’. Her customers wellbeing might not improve, but I suspect her bank account might.
An article in THE TIMES seems worth mentioning. Here are some excerpts:
… Maternity care at Nottingham University Hospitals NHS Trust (NUH) is the subject of an inquiry, prompted by dozens of baby deaths. More than 450 families have now come forward to take part in the review, led by the expert midwife Donna Ockenden. The trust now faces further scrutiny over its use of aromatherapy, after experts branded guidelines at the trust “shocking” and not backed by evidence. Several bereaved families have said they recall aromatherapy being heavily promoted at the trust’s maternity units.
It is being prosecuted over the death of baby Wynter Andrews just 23 minutes after she was born in September 2019. Her mother Sarah Andrews wrote on Twitter that she remembered aromatherapy being seen as “the answer to everything”. Internal guidelines, first highlighted by the maternity commentator Catherine Roy, suggest using essential oils if the placenta does not follow the baby out of the womb quickly enough… the NUH guidelines say aromatherapy can help expel the placenta, and suggest midwives ask women to inhale oils such as clary sage, jasmine, lavender or basil, while applying others as an abdominal compress. They also describe the oils as “extremely effective for the prevention of and, in some cases, the treatment of infection”. The guidelines also suggest essential oils to help women suffering from cystitis, or as a compress on a caesarean section wound. Nice guidelines for those situations do not recommend aromatherapy…
The NUH adds frankincense “may calm hysteria” and is “recommended in situations of maternal panic”. Roy said: “It is shocking that dangerous advice seemed to have been approved by a team of healthcare professionals at NUH. There is a high tolerance for pseudoscience in NHS maternity care … and it needs to stop. Women deserve high quality care, not dangerous quackery.” …
________________________________
The journalist who wrote the article also asked me for a comment, and I emailed her this quote: “Aromatherapy is little more than a bit of pampering; no doubt it is enjoyable but it is not an effective therapy for anything. To use it in medical emergencies seems irresponsible to say the least.” The Times evidently decided not to include my thoughts.
Having now read the article, I checked again and failed to find good evidence for aromatherapy for any of the mentioned conditions. However, I did find an article and an announcement both of which are quite worrying, in my view:
Aromatherapy is often misunderstood and consequently somewhat marginalized. Because of a basic misinterpretation, the integration of aromatherapy into UK hospitals is not moving forward as quickly as it might. Aromatherapy in UK is primarily aimed at enhancing patient care or improving patient satisfaction, and it is frequently mixed with massage. Little focus is given to the real clinical potential, except for a few pockets such as the Micap/South Manchester University initiative which led to a Phase 1 clinical trial into the effects of aromatherapy on infection carried out in the Burns Unit of Wythenshawe Hospital. This article discusses the expansion of aromatherapy within the US and follows 10 years of developing protocols and policies that led to pilot studies on radiation burns, chemo-induced nausea, slow-healing wounds, Alzheimers and end-of-life agitation. The article poses two questions: should nursing take aromatherapy more seriously and do nurses really need 60 hours of massage to use aromatherapy as part of nursing practice?
My own views on aromatherapy are expressed in our now not entirely up-to-date review:
Aromatherapy is the therapeutic use of essential oil from herbs, flowers, and other plants. The aim of this overview was to provide an overview of systematic reviews evaluating the effectiveness of aromatherapy. We searched 12 electronic databases and our departmental files without restrictions of time or language. The methodological quality of all systematic reviews was evaluated independently by two authors. Of 201 potentially relevant publications, 10 met our inclusion criteria. Most of the systematic reviews were of poor methodological quality. The clinical subject areas were hypertension, depression, anxiety, pain relief, and dementia. For none of the conditions was the evidence convincing. Several SRs of aromatherapy have recently been published. Due to a number of caveats, the evidence is not sufficiently convincing that aromatherapy is an effective therapy for any condition.
In this context, it might also be worth mentioning that we warned about the frequent usage of quackery in midwifery years ago. Here is our systematic review of 2012 published in a leading midwifery journal:
Background: in recent years, several surveys have suggested that many midwives use some form of complementary/alternative therapy (CAT), often without the knowledge of obstetricians.
Objective: to systematically review all surveys of CAT use by midwives.
Search strategy: six electronic databases were searched using text terms and MeSH for CAT and midwifery.
Selection criteria: surveys were included if they reported quantitative data on the prevalence of CAT use by midwives.
Data collection and analysis: full-text articles of all relevant surveys were obtained. Data were extracted according to pre-defined criteria.
Main results: 19 surveys met the inclusion criteria. Most were recent and from the USA. Prevalence data varied but were usually high, often close to 100%. Much use of CATs does not seem to be supported by strong evidence for efficacy.
Conclusion: most midwives seem to use CATs. As not all CATs are without risks, the issue should be debated openly.
I am tired of saying ‘I TOLD YOU SO!’ but nevertheless find it a pity that our warning remained (yet again) unheeded!
Two million people in UK are estimated to be currently suffering from long COVID, says the Office for National Statistics. Fatigue continues to be the most common symptom – experienced by 55% of those with self-reported long COVID – followed by 32% with shortness of breath, 23% with a cough, and 23% with muscle ache. The problem is only going to increase in the near future. Thus, many people are frantically looking for an effective therapy. Practitioners of so-called alternative medicine (SCAM) are no exception.
This study aimed to evaluate the potential for inhalation of essential oils to improve energy levels among otherwise healthy female survivors of acute COVID-19 who experience a lack of energy more than five months after recovery.
This was a randomized double-blind, placebo-controlled trial to evaluate the potential for inhalation of Longevity™, a proprietary essential oil blend manufactured by Young Living Essential Oils (Lehi, Utah, USA), on energy levels among female survivors of COVID-19 who continue to experience fatigue more than 5 months recovery from the acute infection. Forty women were randomized to two groups: intervention and placebo. The placebo product contained an inert, odorless fractionated coconut oil. Both groups inhaled the assigned product twice daily for fourteen consecutive days. Fatigue scores were measured using the Multidimensional Fatigue Symptom Inventory (MFSI). Secondary outcomes included scores on each of the MFSI’s ten subscales.
Individuals who inhaled the essential oil blend for 2 weeks had significantly lower fatigue scores after controlling for baseline scores, employment status, BMI, olfactory function, and time since diagnosis, with a large effect size (F (1,39) = 6.15, p = .020, partial eta squared = 0.198). Subscale analysis identified subscales of vigor, as well as global, behavioral, general, and mental fatigue as benefiting from the intervention. This study provides evidence that a proprietary aromatherapy blend can significantly improve energy levels among women who are experiencing fatigue after recovering from COVID-19.
The authors concluded that the use of aromatherapy with Longevity™ essential oil blend to boost energy levels in women who have recovered from COVID-19 provides a novel, non-invasive approach to improving quality of life in this population. This intervention is particularly beneficial for global and mental fatigue, as well as vigor. Other subdomains may experience improvements to energy levels with a smaller effect size; future studies should be conducted to explore this potential.
This trial was funded by Young Living Essential Oils. Perhaps, this explains why there is no mention of the elephant in the room: the trial was not blind! Participants in the verum group knew that they received aromatherapy. Likewise, participants in the placebo group knew that they received the placebo.
Could this fact have influenced the outcome? Certainly!
Could the trial have been designed better? Certainly!
All the investigators needed to do is to use a nice-smelling oil that, according to aromatherapists, does not boost energy, as the placebo.
As it stands, we have no idea whether the authors’ assumption that the verum oil caused the effect is true.
Pity!
Or maybe not?
Perhaps Young Living Essential Oils, the sponsor of the study and producer of the oil never wanted to know the truth. Maybe they are happy to abuse science as a marketing tool?
Aromatherapy, the use of essential oils for medicinal purposes, exists in several guises. One of them is inhalation aromatherapy which is a complementary therapy used in different clinical settings. But is there any sound evidence about its effectiveness?
The aim of this review was to assess the effectiveness of inhalational aromatherapy in the care of hospitalized pediatric patients.
A systematic review of clinical trials and quasi-experimental studies was conducted, based on PRISMA recommendations, searching Medline, Web of Science, Scopus, SciELO, LILACS, CINAHL, Science Direct, EBSCO, and updated databases. The Down and Black 2020, RoB 2020 CLARITY, and ROBINS-I 2020 scales were used through the Distiller SR software to verify the studies’ internal validity and risk of bias.
From 446 articles identified, 9 fulfilled the inclusion criteria. Seven were randomized controlled trials (RCTs), one pilot RCT, and one non-randomized quasi-experimental trial.
Different outcomes were analyzed, with pain being the most frequently measured variable. None of the 6 studies that evaluated pain showed significant effects with inhalation aromatherapy. Additionally, non-significant effects were found regarding nausea, vomiting, and behavioral/emotional variables.
The authors concluded that the findings are still inconclusive, and more evidence is required from future studies with high methodological quality, blinding, and adequate sample sizes.
Inconclusive?
Really?
Call me a skeptic, but I think the findings show quite clearly that there is no sound evidence to suggest that inhalation aromatherapy might be effective for kids.
Psychosocial distress, depression, or anxiety are frequent problems of women after a breast cancer diagnosis and treatment. Many try so-called alternative medicine (SCAM) in an attempt to deal with them. But is this effective?
The purpose of this study was to assess the potential benefit of lavender oil as a perioperative adjunct to improve anxiety, depression, pain, and sleep in women undergoing microvascular breast reconstruction.
This was a prospective, single-blinded, randomized, controlled trial of 49 patients undergoing microvascular breast reconstruction. Patients were randomized to receive lavender oil or a placebo (coconut oil) throughout their period of hospitalization. The effect of lavender oil on perioperative stress, anxiety, depression, sleep, and pain was measured using the hospital anxiety and depression scale, Richards-Campbell Sleep Questionnaire, and the visual analogue scale.
Twenty-seven patients were assigned to the lavender group and 22 patients were assigned to the control group. No significant differences were seen in the perioperative setting between the groups with regard to anxiety (p = 0.82), depression, sleep, or pain scores. No adverse events were noted, and no significant differences in surgery-related complications were observed. When evaluating the entire cohort, postoperative anxiety scores were significantly lower than preoperative scores, while depression scores were significantly higher postoperatively as compared with preoperatively.
The authors concluded that, in the setting of microvascular breast reconstruction, lavender oil and aromatherapy had no significant adverse events or complications; however, there were no measurable advantages pertaining to metrics of depression, anxiety, sleep, or pain as compared with the control group.
One could argue that the sample size of the trial was too low to pick up small differences in the outcome measures. Yet, even then, the findings do not suggest that the treatment did make a large enough difference to justify the effort and expense of the treatment.
One could also argue that – who cares? – if a patient wants aromatherapy (or another SCAM that is harmless), why not? The answer to this is the fact that researchers have the ethical duty to identify the most effective treatment, and clinicians have the ethical duty to employ not just any odd therapy but the one that works demonstrably best. Seen from this perspective, the place of SCAM in cancer care seems far less certain than many enthusiasts try to make us believe.