naturopathy
We all have heard of so-called alternative therapies but few of us are aware of the fact that there are also alternative diagnoses. These are diagnoses used regularly by practitioners of so-called alternative medicine (SCAM) that have no basis on science, or – to put it simply – that do not exist. They are nonetheless popular with SCAM practitioners and allegedly cause a wide range of non-specific symptoms such as:
- anxiety,
- brain fog,
- constipation,
- depression,
- dizziness,
- fatigue,
- headaches,
- heart palpitations,
- insomnia,
- irritability,
- muscle and joint pain,
- loss of appetite,
- loss of libido,
- weight gain.
In this series of posts, I will briefly discuss some of these diagnoses and list the treatments that SCAM practitioners might recommend for them.
Adrenal Fatigue
Adrenal fatigue is not the same as adrenal insufficiency or Addison’s disease; it is a term coined by a chiropractor who claimed that the stresses of modern life tire out the adrenal glands. In turn, this phenomenon allegedly leads to generalised weariness.
There is not evidence that this is true, nor that adrenal fatigue even exists. A systematic review of the evidence concluded that “there is no substantiation that adrenal fatigue is an actual medical condition.”
Yet, SCAM practitioners advise to cure adrenal fatigue with a range of dietary supplements (e.g. fish oil, ashwagandha, rhodiola rosea, schisandra and holy basil, licorice, magnesium, various vitamins), special diets, lifestyle adjustments, stress management and many other SCAMs. They all have in common that their effectiveness is not supported by convincing evidence from rigorous clinical trials.
Candidiasis hypersensitivity
Most of us are infected by the fungus Candida albicans without being affected by it in any way. Yet, many SCAM practitioners claim that candidiasis hypersensitivity is a condition that causes symptoms like fatigue, premenstrual tension, gastrointestinal symptoms, and depression and therefore needs treating.
But, candidiasis hypersensitivity does not exist. An RCT concluded that, “in women with presumed candidiasis hypersensitivity syndrome, nystatin does not reduce systemic or psychological symptoms significantly more than placebo.”
This, however, does not stop SCAM practitioners to recommend numerous forms of SCAM to treat the condition, e.g.: dietary supplements containing probiotics, milk thistle, red thyme, barberry, garlic, or external applications of coconut oil, essential oils of peppermint oil, lavender oil, oregano oil, and tea tree. No sound evidence exists to show that ant of these SCAMs can successfully treat the condition.
Chronic intoxications
Chronic intoxications do ecist, of course. But in the realm of SCAM, they are diagosed for the sole putpose of selling their various ‘detox’ treatments. The alleged rationale is that our bodies are overloaded with all sorts ot harmful substances, for instance, from the environment, from our food, from modern drugs, or from our own metabolism.
To eliminate them, we need to ‘detox’. For that purpose, SCAM practitioners recommend a very wide range of SCAMs; in fact, it is hardly possible to identify a single form of SCAM that is not said to detoxify our bodies. Yet, for none of them is there compelling evidence that it eliminates toxins from our body. Some of the most popular detox regimen include:
- acupuncture;
- CEASE therapy;
- chelation therapy;
- crystal healing;
- cupping;
- detox diets;
- detox supplements;
- gua sha;
- homeopathy;
- homotoxicology;
- Kombucha;
- oil pulling;
- vaginal steaming.
Interim conclusion: non-existing diagnoses are perfect opportunities for SCAM practitioners to rip off gullible patients.
Rheumatoid arthritis (RA) is a chronic, systemic, polyarticular autoimmune inflammatory disease that destroys the capsule and synovial lining of joints. Antirheumatic treatment reduces disease activity and inflammation, but not all patients respond to treatment. Naturopathy is claimed to be effective, but there is little data on the effect on inflammation and disease activity in RA. The objective of this study was therefore to explore the effect of 12 weeks of integrated naturopathy interventions on disease-specific inflammatory markers and quality of life in RA patients.
A total of 100 RA patients were randomized into two groups:
- the naturopathy group (integrated naturopathy interventions with routine medical therapy),
- the control group (only with routine medical therapy).
Blood samples were collected pre- and post-intervention for primary outcome measurements of systemic inflammatory markers (ESR, CRP, and IL-6). Disease activity score (DAS-28) and quality of life were used to assess disease activity and functional status using SF-36, respectively, at pre- and post-intervention time points.
The results show a notable decrease in disease activity after 12 weeks of naturopathy intervention. As such, a significant decrease was found in levels of systemic inflammatory markers such as ESR (p = 0.003) and IL-6 (p < 0.001), RA disease activity score (DAS-28) (p = 0.02), and most of the components of health-related quality of life (SF 36 scores) (p < 0.05) except in vitality (p = 0.06).
The authors conclused that the findings of the present study suggest that integrated naturopathy treatments may have the ability to control persistent inflammation, maintain immune homeostasis, and lower disease activity.
The naturopathic treatments included:
- acupuncture,
- hot and cold-water application to the painful joints,
- sauna baths,
- enemas,
- fasting,
- mud therapy,
- massage therapy.
The study was designed as an A+B versus B trial. Therefore, it is hardly surprising that subjective endpoints improved. A little more baffling are the changes in objective parameters. These could easily be due to the fasing interventions – there is reasonably sound evidence for such effects. Take this review, for instance:
Fasting is an act of restricting, for a certain length of time, food intake or intake of particular foods, and has been part of religious rituals for centuries. Religions such as Christianity and Islam use this practice as a form of sacrifice, self-discipline, and gratitude. However, in the past decade, fasting has penetrated the mainstream as a diet trend. There are several ways of fasting; existing fast mimicking eating methods promise accelerated weight loss, and many more benefits: lower cholesterol, prevention of type 2 diabetes and a longer lifespan. Even more, it has been proposed that fasting can downregulate the inflammatory process and potentially be used as a treatment regimen for several diseases. Here, we review the effects of fasting on immune and inflammatory pathways. Also, we present current knowledge about the role of fasting in the activity of inflammatory arthritides with a focus on rheumatoid arthritis.
What I am trying to say is this: some modalities used in naturopathy might well be effective in treating certain conditions. In my view, this is, however, no reason for condoning or recommening naturopathy as a whole. Or – to put it bluntly – naturopathy is a weird mixture of pure nonsense and some possibly reasonable interventions.
It has been reported that 5 people who took a Japanese health supplement have died and more than 100 have been hospitalized as of Friday, a week after a pharmaceutical company issued a recall of the products, officials said. Osaka-based Kobayashi Pharmaceutical Co. came under fire for not going public quickly with problems known internally as early as January. Yet the first public announcement came only on 22 March. Company officials said 114 people were being treated in hospitals after taking products — including Benikoji Choleste Help meant to lower cholesterol — that contain an ingredient called benikoji, a red species of mold. Some people developed kidney problems after taking the supplements, but the exact cause was still under investigation in cooperation with government laboratories, according to the manufacturer.
“We apologize deeply,” President Akihiro Kobayashi told reporters last Friday, bowing for a long time to emphasize the apology alongside three other top company officials. He expressed remorse to those who have died and have been taken ill and to their families. He also apologized for the troubles caused to the entire health food industry and the medical profession, adding that the company was working to prevent further damage and improve crisis management.
The company’s products have been recalled — as have dozens of other products that contain benikoji, including miso paste, crackers, and a vinegar dressing. Japan’s health ministry put up a list on its official site of all the recalled products, including some that use benikoji for food coloring. The ministry warned the deaths could keep growing. The supplements could be bought at drug stores without a prescription from a doctor, and some may have been purchased or exported before the recall, including by tourists who may not be aware of the health risks.
Kobayashi Pharmaceutical had been selling benikoji products for years, with a million packages sold over the past 3 fiscal years, but a problem crept up with the supplements produced in 2023. Kobayashi Pharmaceutical said it produced 18.5 tons of benikoji last year. Some analysts blame the recent deregulation initiatives, which simplified and sped up approval for health products to spur economic growth.
________________________
Anouther source reported that Japanese authorities on Saturday raided a drug factory after a pharmaceutical company reported at least five deaths and 114 hospitalizations possibly linked to a health supplement. About a dozen Japanese health officials walked into the Osaka plant of the Kobayashi Pharmaceutical Co., as seen in footage of the raid widely telecasted on Japanese news. The health supplement in question is a pink pill called Benikoji Choleste Help. It is said to help lower cholesterol levels. A key ingredient is benikoji, a type of red mold. The company has said it knows little about the cause of the sickness, which can include kidney failure. It is currently investigating the effects in cooperation with Japan’s government.
___________________________
More recent reports update the figure of affected individuals: Japanese dietary supplements at the center of an expanding health scare have now been linked to at least 157 hospitalizations, a health ministry official said Tuesday.The figure reflects an increase from the 114 hospitalization cases that Kobayashi Pharmaceutical said on Friday were linked to its products containing red yeast rice, or beni kōji.
A Kobayashi Pharmaceutical spokeswoman confirmed the latest hospitalization cases without elaborating further.
Benikoji is widely sold and used; not just in Japan. It comes under a range of different names:
- red yeast rice,
- red fermented rice,
- red kojic rice,
- red koji rice,
- anka,
- angkak,
- Ben Cao Gang Mu.
It is a bright reddish purple fermented rice which acquires its color from being cultivated with the mold Monascus purpureus. Red yeast rice is used as food and as a medicine in Asian cultures, such as Kampo and TCM.
It contains lovastatin which, of course, became patented and is marketed as the prescription drug, Mevacor. Red yeast rice went on to become a non-prescription dietary supplement in the United States and other countries. In 1998, the U.S. FDA banned a dietary supplement containing red yeast rice extract, stating that red yeast rice products containing monacolin K are identical to a prescription drug, and thus subject to regulation as a drug.
The Amercian Medical Association (AMA) recently published a lengthy article on naturopathy in the US. Here are some excerpts:
There are three types of health professionals who offer naturopathic treatment:
- Naturopathic doctors. These nonphysicians graduate from a four-year, professional-level program at an accredited naturopathic medical school, earning either the doctor of naturopathy (ND) degree or the doctor of naturopathic medicine (NMD) degree.
- Traditional naturopaths, who have obtained education through some combination of a mentorship program with another professional or at an alternative clinic, distance-learning program or classroom schooling on natural health, or other holistic studies.
- Other health professionals such as chiropractors, massage therapists, dentists, nurses, nutritionists, or physicians who practice under a professional license but include some naturopathic methods in their practice and who may have studied on their own or taken courses on naturopathic methods.
At least 24 states and the District of Columbia regulate the practice of naturopathy. In order to be licensed, naturopaths in these states must earn an ND or NMD from an accredited naturopathic program and pass the Naturopathic Physicians Licensing Exam. Three states—Florida, South Carolina and Tennessee—prohibit the practice of naturopathy. In states that neither license nor prohibit the practice of naturopathy, traditional naturopaths and NDs alike may practice without being subject to state regulation.
Postgraduate training is neither common nor required of graduates of naturopathic schools, except in Utah … less than 10% of naturopaths participate in an approved residency, and such residencies last only a year and lack a high degree of standardization.
… naturopaths are required to get at least 1,200 hours of direct patient contact, physicians get 12,000–16,000 hours of clinical training…
ND programs emphasize naturopathic principes—for example, the healing power of nature—and naturopathic therapeutics such as botanical medicine, homeopoathy and hydrotherapy. Coursework in naturopathic therapeutics is combined with, and taught alongside, coursework in sciences. But there are no specifications around the number of hours required in each area … naturopathic students may lack exposure to key clinical scenarios in the course of their training … naturopathic students’ clinical experience is typically gained through outpatient health care clinics, as naturopathic medical schools typically do not have significant hospital affiliation. This means there is no guarantee that a naturopathic student completing a clinical rotation will see patients who are actually sick or hospitalized, and they may not be exposed to infants, children, adolescents or the elderly. It has been said that naturopaths tend to treat the “worried well.”
… Naturopaths claim they are trained as primary care providers and, as such, are educated and trained to diagnose, manage and treat many conditions, including bloodstream infections, heart disease and autoimmune disorders. Yet their education and training falls several years and thousands of hours short of what physicians get.
…The AMA believes it is the responsibility of policymakers to ensure that naturopaths’ claims that they can treat a broad range of conditions are backed by facts—facts that include the specific education and training necessary to ensure patient safety.
________________
The AMA is clearly cautious here. A less polite statement might simply stress that naturopaths are taught a lot of nonsense which they later tend to administer to their unsuspecting patients. On this blog, we have repeatedly discussed the danger naturopaths present to public health in the US and elsewhere, e.g.:
- How reliable are the claims made by naturopathic influencers?
- Naturopath jailed for selling fraudulent vaccination documents
- Naturopath fined for misdiagnosing and treating a rectal tumor for hemorrhoids
- Naturopaths are ‘not bound by science,’ lawyer argues
- Vaccination rates of Canadian healthcare professionals: those of chiropractors and naturopaths are at the lowest
- Is veterinary naturopathy animal abuse?
- Naturopathic ‘cancer specialist’ using coffee enemas found guilty
- Patients consulting chiropractors, homeopaths, or naturopaths are less likely to agree to the flu jab
- A naturopath responsible for the death of two cancer patients was sentenced to two years
- A naturopath in court after two of his cancer patients died
- Many naturopaths, homeopaths, and chiropractors are a risk to public health
- Naturopath treats autism with fecal transplants
- A naturopath promoting fake news about COVID vaccinations
- Naturopathy (according to the WNF) = quackery steeped in obsolete fantasies
- Canadian naturopaths may no longer call themselves ‘medically trained’
- Naturopaths’ counselling against vaccinations could be criminally negligent
- Naturopathy for cancer … claims that have the potential to be lethal
- Severe liver injury due to naturopaths’ prescription of Epsom salt
- Naturopaths should not treat children
- Some naturopaths are clearly a danger to public health
- Death of a child through naturopathy
Claims that naturopaths are a viable alternative to evidence-based medicine are wrong, irresponsible and dangerous. Regulators must be reminded that they have the duty to protect the public from charlatans and should therefore ensure that no false therapeutic or diagnostic claims can be made by naturopaths.
Looking at some ancient papers of mine, I came across a short BMJ paper from 1994. Here is a passage from it:
… A standard letter (on departmental letterhead) was written (in German) to all 189 firms that we identified as marketing herbal drugs in Germany. It asked (among other questions) for reprints of articles reporting controlled clinical trials on the company’s product(s).
Only 19 replies had reached us six weeks later. Four of these included at least one reprint. Twelve respondents regretted not knowing of clinical trials on their drug(s). In three cases we had written to a wrong address (one
instance) or to a firm which did not market phytomedicines (two instances).
These data, though far from conclusive, do not give the impression that research is in proportion to either prevalence or financial tumover of herbal remedies…
I wonder what the results would be, if we repeated this little excercise today, 30 years afteer the original investigation. I fear that the findings would be much the same or perhaps even worse. I also suspect that they would be similar regardless of the country we chose. Those who sell herbal remedies have very little incentive to do expensive clinical trials to test whether the products they earn their money with actually work. They may be doing well without it and ask themselves, why spend money on research that might not show what we hope and could easily turn out to jeopardize our financial success?
But the problem is by no means confined to herbal manufacturers (who would arguably have an important share to initiate and sponsor research). Even though fundamental questions remain unanswered, research into herbal medicine is scarce across the board.
To see whether this statement is true, I did a very quick Medline search. It showed that, in 2023, just over 13 000 papers on herbal medicine emerged. Of those, just 460 were listed as clinical trials. The latter figure is almost certainly considerably smaller than the true amount because Medline is over-generous in classifying papers as clinical trials. I thus estimate that only around 200 clinical trials of herbal medicine are conducted each year. Considering that we are dealing with thousands of herbs and ten thousands of herbal products, this figure is an embarrassment for the sector – which, as we have seen just days ago, is doing extremely well in finacial terms.
Dry needling is a therapy that is akin to acupuncture and trigger point therapy. It is claimed to be safe – but is this true?
Researchers from Ghent presented a series of 4 women aged 28 to 35 who were seen at the emergency department (ED) with post-dry needling pneumothorax between September 2022 and December 2023. None of the patients had any relevant medical history. All had been treated for a painful left shoulder, trapezius muscle or neck region in outpatient physiotherapist practices. At least three different physiotherapists were involved.
One patient presented to the ER on the same day as the dry needling procedure, the others presented the day after. All mentioned thoracic pain and dyspnoea. Clinical examination in all of these patients was unremarkable, as were their vital signs. Diagnosis was confirmed with ultrasound (US) and chest X-ray (CXR) in all patients. The latter exam showed left-sided apical pleural detachment with a median of 3.65 cm in expiration.
Two patients were managed conservatively. One patient (initial pneumothorax 2.5 cm) was discharged. The US two days later displayed a normally expanded lung. One patient with an initial apical size of 2.8 cm was admitted with 2 litres of oxygen through a nasal canula and discharged from the hospital the next day after US had shown no increase in size. Her control CXR 4 days later showed only minimal pleural detachment measuring 6 mm. The two other patients were treated with US guided needle aspiration. One patient with detachment initially being 4.5 cm showed decreased size of the pneumothorax immediately after aspiration. She was admitted to the respiratory medicine ward and discharged the next day. Control US and CXR after 1 week showed no more signs of pneumothorax. In the other patient, with detachment initially being 5.5 cm, needle aspiration resulted in complete deployment on US immediately after the procedure, but control CXR showed a totally collapsed lung 3 hours later. A small bore chest drain was placed but persistent air leakage was seen. Several trials of clamping the drain resulted in recurrent collapsing of the lung. After CT-scan had shown no structural deformities of the lung, suction was gradually reduced and the drain was successfully removed on the sixth day after placement. The patient was then discharged home. Control CXR 3 weeks later was normal.
The authors concluded that post-dry needling pneumothorax is, contrary to numbers cited in literature, not extremely rare. With rising popularity of the technique we expect complications to occur more often. Patients and referring doctors should be aware of this. In their informed consent practitioners should mention pneumothorax as a considerable risk of dry needling procedures in the neck, shoulder or chest region.
The crucial question, in my view, is this: do the risks of dry-needling out weigh the risks of this form of therapy? Let’s have a look at some of the recent evidence that we discussed on this blog:
- Spinal Manipulation and Electrical Dry Needling for Subacromial Pain Syndrome: A Nonsensical Trial
- Dry needling is useless for rehabilitation after shoulder surgery
- High velocity, low amplitude techniques are not superior to no treatment in the management of tension-type headache
- Which treatments are best for acute and subacute mechanical non-specific low back pain? A systematic review with network meta-analysis
- Acupuncture for chronic pain: the new NICE guideline
- Acupuncture for the Relief of Chronic Pain? A new, thorough synthesis fails to produce strong evidence that acupuncture works
The evidence is clearly mixed and unconvincing. I am not sure whether it is strong enough to afford a positive risk/benefit balance. In other words: dry needling is a therapy that might best be avoided.
I usually take ‘market reports’ with a pinch of salt. Having said that, this document makes some rather interesting predictions:
The size of the market for so-called alternative medicine (SCAM) is projected to expand from USD 147.7 billion in 2023 to approximately USD 1489.4 billion by the year 2033. This projection indicates a remarkable Compound Annual Growth Rate (CAGR) of 26% over the forecast period.
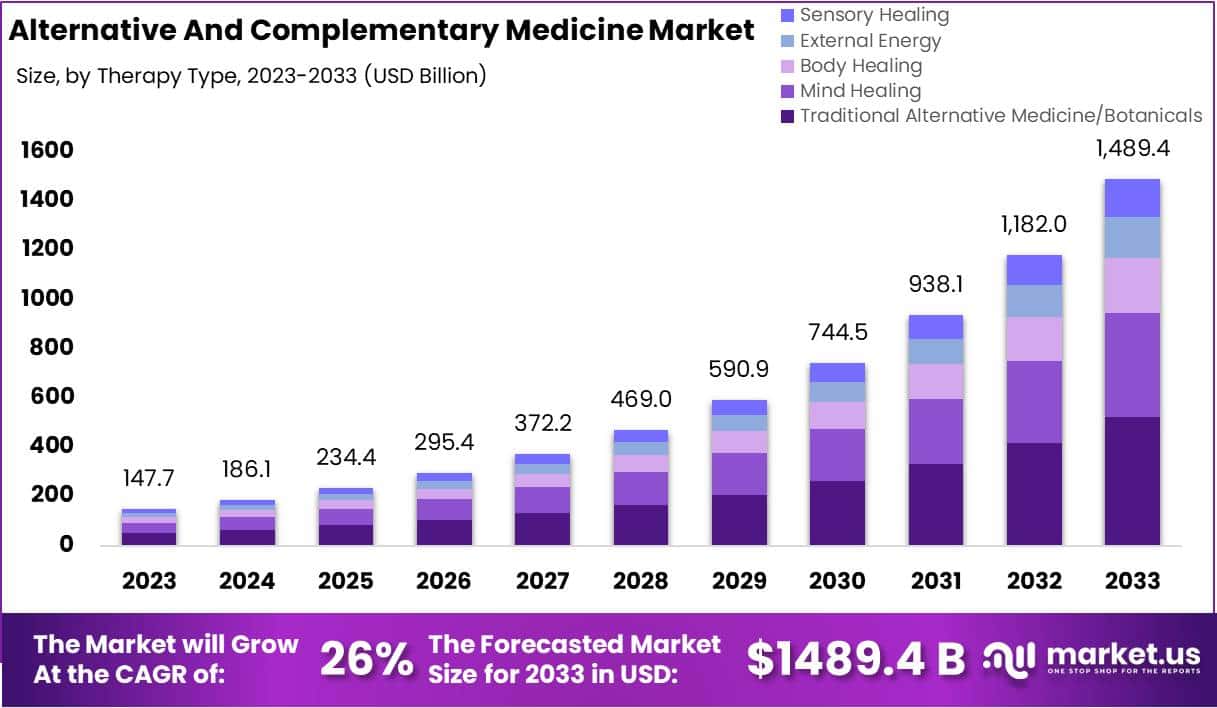
The market for SCAM is experiencing significant growth, fueled by increasing consumer interest in natural and holistic health solutions. This trend reflects a broader shift in societal attitudes towards health and wellness, emphasizing preventive care and natural health practices.
The market’s dynamics are influenced by various factors, including consumer preferences, regulatory standards, and evolving perceptions of health and wellness. As the popularity of these alternative therapies grows, it is crucial for individuals to consult with healthcare professionals to ensure that these non-conventional approaches are safely and effectively incorporated into their overall health regimen. The increasing acceptance of SCAM underscores a collective move towards more personalized and holistic healthcare solutions, resonating with today’s health-conscious consumers.
In 2023, Traditional Alternative Medicine/Botanicals led the market, capturing a 35.2% share, which reflects a strong consumer inclination towards these treatments. Dietary Supplements were prominent in the market, securing a 25.1% share in 2023, which underscores the high consumer demand for nutritional aids. Direct Sales were the most favored distribution channel, accounting for 43.2% of the market share in 2023, which indicates their significant impact on guiding consumer purchases. Pain Management was the predominant application area, holding a 24.9% market share in 2023, propelled by the growing acknowledgment of non-pharmacological treatment options. Adults represented a substantial portion of the market, making up 62.33% in 2023, signifying a marked preference for SCAM therapies within this age group. Europe stood out as the market leader, claiming a 42.6% share in 2023, a position supported by widespread acceptance, governmental backing, and an increasing elderly population. The regions of North America and Asia-Pacific are highlighted as areas with potential, signaling opportunities for market expansion beyond the European stronghold in the upcoming years.
Leading Market Players Are:
- Columbia Nutritional
- Nordic Nutraceuticals
- Ramamani Iyengar Memorial Yoga Institute
- The Healing Company Ltd.
- John Schumacher Unity Woods Yoga Centre
- Sheng Chang Pharmaceutical Company
- Pure encapsulations LLC.
- Herb Pharm
- AYUSH Ayurvedic Pte Ltd.
Recent developments:
- In December 2023, Adoratherapy launched the Alkemie Chakra Healing Line, an aromatherapy range aimed at harmonizing the seven chakras.
- Coworth Park introduced the Hebridean Sound Treatment in October 2023, merging traditional Hebridean sounds with guided meditation to offer a novel, restorative wellness experience.
- The World Health Organization released draft guidelines in September 2023 for the safe, effective application of traditional medicines.
- Telehealth services, expanding significantly in August 2023, have broadened the reach of SCAM, enhancing patient access to these treatments.
Here is the abstract of a recent article that I find worrying:
In 2020, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) challenged the world with a global outbreak that led to millions of deaths worldwide. Coronavirus disease 2019 (COVID-19) is the symptomatic manifestation of this virus, which can range from flu-like symptoms to utter clinical complications and even death. Since there was no clear medicine that could tackle this infection or lower its complications with minimal adverse effects on the patients’ health, the world health organization (WHO) developed awareness programs to lower the infection rate and limit the fast spread of this virus. Although vaccines have been developed as preventative tools, people still prefer going back to traditional herbal medicine, which provides remarkable health benefits that can either prevent the viral infection or limit the progression of severe symptoms through different mechanistic pathways with relatively insignificant side effects. This comprehensive review provides scientific evidence elucidating the effect of 10 different plants against SARS-CoV-2, paving the way for further studies to reconsider plant-based extracts, rich in bioactive compounds, into more advanced clinical assessments in order to identify their impact on patients suffering from COVID-19.
The conclusions of this paper read as follows:
…since these 10 herbs hold distinct bioactive compounds with significant properties in vitro and with remarkable benefits to human health, it is possible to prevent SARS-CoV-2 infection and reduce its symptomatic manifestations by consuming any of these 10 plants according to the recommended dose. The diversity in bioactive molecules between the different plants exerts various effects through different mechanisms at once, which makes it more potent than conventional synthetic drugs. Nonetheless, more studies are needed to highlight the clinical efficacy of these extracts and spot their possible side effects on patients, especially those with comorbidities who take multiple conventional drugs.
I should point out that the authors fail to offer a single reliable trial that would prove or even imply that any of the 10 herbal remedies can effectively treat or prevent COVID infections (to the best of my knowledge, no such studies exist). Laguage like “it is possible to prevent SARS-CoV-2 infection and reduce its symptomatic manifestations” is therefore not just misleading but highly dangerous and deeply unethical. Sadly, such evidence-free claims abound in herbal medicine.
I think the journal editor, the peer-reviewer, the authors and their universities ( University of Tripoli in Lebanon, American University of the Middle East, Egaila in Kuwait, University of Balamand, Kalhat, Tripoli in Lebanon, Lebanese University, Tripoli in Lebanon, Aix-Marseille Université in France) should be ashamed to produce such dangerous rubbish.
Traditional herbal medicine (THM) is frequently used in pediatric populations. This is perticularly true in many low-income countries. Yet THM has been associated with a range of adverse events, including liver toxicity, renal failure, and allergic reactions. Despite these concerns, its impact on multi-organ dysfunction syndrome (MODS) risk has so far not been thoroughly investigated.
This study aimed to investigate the incidence and predictors of MODS in a pediatric intensive care unit (PICU) in Ethiopia, with a focus on the association between THM use and the risk of MODS. It was designed as a single-center prospective cohort study conducted at a PICU in the university of Gondar Comprehensive Specialized hospital, Northwest Ethiopia. The researchers enrolled eligible patients aged one month to 18 years admitted to the PICU during the study period. Data on demographic characteristics, medical history, clinical and laboratory data, and outcome measures using standard case record forms, physical examination, and patient document reviews. The predictors of MODS were assessed using Cox proportional hazards models, with a focus on the association between traditional herbal medicine use and the risk of MODS.
A total of 310 patients were included in the final analysis, with a median age of 48 months and a male-to-female ratio of 1.5:1. The proportion and incidence of MODS were 30.96% (95% CI:25.8, 36.6) and 7.71(95% CI: 6.10, 9.40) per 100-person-day observation respectively. Renal failure (17.74%), neurologic failure (15.16%), and heart failure (14.52%) were the leading organ failures identified. Nearly one-third of patients (32.9%) died in the PICU, of which 59.8% had MODS. The rate of mortality was higher in patients with MODS than in those without. The Cox proportional hazards model identified renal disease (AHR = 6.32 (95%CI: 3.17,12.61)), intake of traditional herbal medication (AHR = 2.45, 95% CI:1.29,4.65), modified Pediatric Index of Mortality 2 (mPIM 2) score (AHR = 1.54 (95% CI: 1.38,1.71), and critical illness diagnoses (AHR = 2.68 (95% CI: 1.77,4.07)) as predictors of MODS.
The authors concluded that the incidence of MODS was high. Renal disease, THM use, mPIM 2 scores, and critical illness diagnoses were independent predictors of MODS. A more than twofold increase in the risk of MODS was seen in patients who used TMH. Healthcare providers should be aware of risks associated with THM, and educate caregivers about the potential harms of these products. Future studies with larger sample sizes and more comprehensive outcome measures are needed.
I do fully agree with the authors about the high usage of herbal and other so-called alternative medicines by children. We have shown that, in the UK the average one-year prevalence rate was 34% and the average lifetime prevalence was 42%. We have furthermore shown that the evidence base for these treatments in children is weak, even more so than for general populations. Finally, we can confirm that adverse effects are far from rare and often serious.
It is therefore high time, I think, that national regulators do more to protect children from SCAM practitioners who are at best uncritical about their treatments and at worse outright dangerous.
The aim of this systematic review and network meta-analysis was to identify the optimal dose and modality of exercise for treating major depressive disorder, compared with psychotherapy, antidepressants, and control conditions.
The screening, data extraction, coding, and risk of bias assessment were performed independently and in duplicate. Bayesian arm based, multilevel network meta-analyses were performed for the primary analyses. Quality of the evidence for each arm was graded using the confidence in network meta-analysis (CINeMA) online tool. All randomised trials with exercise arms for participants meeting clinical cut-offs for major depression were included.
A total of 218 unique studies with a total of 495 arms and 14 170 participants were included. Compared with active controls (eg, usual care, placebo tablet), moderate reductions in depression were found for
- walking or jogging,
- strength training,
- mixed aerobic exercises,
- and tai chi or qigong.
The effects of exercise were proportional to the intensity prescribed. Strength training and yoga appeared to be the most acceptable modalities. Results appeared robust to publication bias, but only one study met the Cochrane criteria for low risk of bias. As a result, confidence in accordance with CINeMA was low for walking or jogging and very low for other treatments.
The authors concluded that exercise is an effective treatment for depression, with walking or jogging, yoga, and strength training more effective than other exercises, particularly when intense. Yoga and strength training were well tolerated compared with other treatments. Exercise appeared equally effective for people with and without comorbidities and with different baseline levels of depression. To mitigate expectancy effects, future studies could aim to blind participants and staff. These forms of exercise could be considered alongside psychotherapy and antidepressants as core treatments for depression.
As far as I can see, there are two main problems with these findings:
- Because too many of the studies are less than rigorous, the results are not quite as certain as the conclusions would seem to imply.
- Patients suffering from a major depressive disorder are often unable (too fatigued, demotivated, etc.) to do and/or keep up vigorous excerise over any length of time.
What I find furthermore puzzling is that, on the one hand, the results show that – as one might expect – the effects are proportional to the intensity of the excercise but, on the other hand tai chi and qugong which are both distinctly low-intensity turn out to be effective.
Nonetheless, this excellent paper is undoubtedly good news and offers hope for patients who are in desperate need of effective, safe and economical treatments.
