Ayurvedic medicine
The BBC has repeatedly misled the public on matters related to so-called alternative medicine (SCAM). Examples include:
- Dangerous BS from the BBC
- The BBC, Michael Mosely, air ionization, depression, and an appalling lack of critical thinking.
Recently the BBC published an article about Ashwagandha. Here it is in its untouched beauty:
Ashwagandha is a herb (Withania somnifera) in the nightshade family, which also includes tomatoes and chilli peppers. It has been used in traditional Indian medicine (Ayurveda) for thousands of years to make preparations for treating various ailments, from infectious diseases, like tuberculosis, to pain and inflammation, baldness and hiccups. In classic Ayurvedic texts, it’s also described as a ‘mental strength promoter’ (or ‘Balya’).
While lots of research has been done on ashwagandha, studies for specific conditions can be sparser. Perhaps the most recent assessment of its use for stress and anxiety comes from a 2022 review of studies by the Cochrane Collaboration, which is internationally recognised for its high-standard medical reviews. Although the Cochrane researchers were only able to find 12 studies on the subject, which together tested the herb on just 1,002 participants, their findings did suggest that ashwagandha can lower stress and anxiety. The researchers rated the ‘certainty’ of the evidence as ‘low’ and called for more detailed studies, though.
The benefits of ashwagandha are thought to be related to natural steroids called withanolides, but this group includes hundreds of compounds, with tens having been isolated from ashwagandha so far. As with any herbal remedy, the combination of compounds and the exact concoction you get depends on how and where the plant is grown, and how it’s prepared. This means that not all supplements based on the same plant are equal.
Remember, too, that herbal doesn’t mean risk-free. For some people, ashwagandha causes drowsiness and more serious side effects aren’t unknown. It’s best to treat it like a drug and not ‘just’ a herb.
The review cited in the article is this one:
Clinical trial studies revealed conflicting results on the effect of Ashwagandha extract on anxiety and stress. Therefore, we aimed to evaluate the effect of Ashwagandha supplementation on anxiety as well as stress. A systematic search was performed in PubMed/Medline, Scopus, and Google Scholar from inception until December 2021. We included randomized clinical trials (RCTs) that investigate the effect of Ashwagandha extract on anxiety and stress. The overall effect size was pooled by random-effects model and the standardized mean difference (SMD) and 95% confidence interval (CIs) for outcomes were applied. Overall, 12 eligible papers with a total sample size of 1,002 participants and age range between 25 and 48 years were included in the current systematic review and meta-analysis. We found that Ashwagandha supplementation significantly reduced anxiety (SMD: −1.55, 95% CI: −2.37, −0.74; p = .005; I2 = 93.8%) and stress level (SMD: −1.75; 95% CI: −2.29, −1.22; p = .005; I2 = 83.1%) compared to the placebo. Additionally, the non-linear dose–response analysis indicated a favorable effect of Ashwagandha supplementation on anxiety until 12,000 mg/d and stress at dose of 300–600 mg/d. Finally, we identified that the certainty of the evidence was low for both outcomes. The current systematic review and dose–response meta-analysis of RCTs revealed a beneficial effect in both stress and anxiety following Ashwagandha supplementation. However, further high-quality studies are needed to firmly establish the clinical efficacy of the plant.
This review is NOT a Cochrane Review; what is more (and more important), it seem rather uncritical.
The BBC article seems to down-play the safety issue related to Ashwagandha. As we have discussed on this blog, Ashwagandha is far from harmless. In fact, Ashwagandha has been shown to be a herb with a high risk of hepatobiliary toxicity as well as heart problems.
So, why does the BBC misinform the public?
Search me.
The BBC stands for reliable information, at least that’s what I used to believe. After reading a recent article published on the BBC website, I have my doubts, however. See for yourself; here are a few excerpts:
On a holiday to Kerala on India’s south-western Malabar Coast, Shilpa Iyer decided to visit Kotakkal, a town that became famous after the establishment of Arya Vaidya Sala, Kerala’s best-known centre for the practice of Ayurveda, in 1902. Seven days later, she left the historical treatment centre after completeing panchakarma, a cleansing and rejuvenating programme for the body, mind and consciousness.
“There was nothing really wrong, but I was always busy with the demands of modern life and plagued with continual aches and pains. So, I decided to focus on my own health,” Iyer says.
Panchakarma, a holistic Ayurvedic therapy, involves a series of detoxifying procedures. It integrates herbal medicines, cleansing therapies, personalised diet plans and wellness activities to eliminate the root cause of disease, revive and rejuvenate the body, and ensure health and longevity.
Iyer says she left “feeling lighter, healthier and better than ever before”. She isn’t the only one who signed up for an Ayurvedic treatment in Kerala; the holistic system of medicine is a way of life in this coastal paradise.
… Ayurveda translates to “knowledge of life” and originated in India more than 3,000 years ago. It is based on the ideology that health and wellness depend on a delicate balance between the mind, body, spirit and environment, and places great emphasis on preventive strategies rather than curative ones. The ancient system of medicine is centred on the idea of universal interconnectedness between prakriti (the body’s constitution) and doshas (life forces). Varied combinations of the five elements — aakash (sky), jal (water), prithvi (earth), agni (fire) and vayu (air) – create the three doshas.

Dr Gaurang Paneri, an Ayurveda practitioner, explains every person has the three doshas, vata, pitta and kapha, in varying strength and magnitude. “The predominant dosha determines their prakriti. Diseases arise when doshas are affected because of an external or internal stimulus (typically linked to eating habits, lifestyle or physical exercise). Ayurveda works to ensure harmony between the three,” he says…
The small state has more than 100 Ayurvedic government-run hospitals, 800 Ayurvedic pharmaceutical factories and 800 Ayurvedic medicine dispensaries. As many as 120 holiday resorts and private wellness centres offer specialised treatments such as kasti vvasti, an oil-based treatment for back pain and inflammation in the lumbosacral region; elakkizhi, a treatment with heated herbal poultices to tackles aches, pains and muskoskeletal trauma; njavara kizhi, a massage therapy for arthritis or chronic musculoskeletal discomfort; and shirodhara, a restorative therapy to ease stress and anxiety and that involves pouring warm, medicated oil over the forehead.
Most treatment centres offer therapies and treatments for a range of health issues, including immunity, mental health, anxiety, pain management, weight loss, skin and health care, sleep issues, psoriasis, eczema, eye care, arthritis, sciatica, gastric problems and paralysis. The treatments typically include dietary changes, herbal medicines, massage therapies, poultices, meditation and breath exercises…
___________________________
I find such advertisements disguised as journalism disturbing:
- No mention that the treatments in question lack conclusive evidence of effectiveness.
- Not a word about the fact that many can be outright dangerous.
- No mention of the often exorbitant fees visitors are asked to pay.
Please do better next time you report about health matters, BBC!
A recent article about ayurvedic medicine caught my eye. Here are a few excerpts:
Imagine if there were a magic pill to ward off COVID-19. Or if you could cure diabetes with vegetable juices and herbal pills instead of controlling it with insulin medication. Or if yoga and breathing exercises were all you need to do to get rid of asthma. These are all claims made by Patanjali Ayurved, one of India’s biggest manufacturers of traditional ayurvedic products…
Many scientists have expressed concerns over the lack of research into the safety and efficacy of ayurvedic products… Nonetheless, Ayurveda enjoys widespread acceptance among Indians. And under India’s Hindu-nationalist government that took power in 2014, ayurveda and other alternative systems of medicine have received unprecedented government support. India’s ministry of alternative medicine gets nearly $500 million a year. The government also promotes ayurveda through its international trade and diplomatic channels. All this set Patanjali’s fortunes soaring.
But now the Supreme Court of India has temporarily banned Patanjali – named after a Hindu mystic best known for his writings on yoga – from advertising some of its products… “The entire country has been taken for a ride,” Ahsanuddin Amanullah, one of the two judges conducting the court hearing, told the lawyer representing the government… The Indian Medical Association had brought the case to court in August 2022, claiming that Patanjali and its brand ambassador Baba Ramdev made a series of false claims against evidence-backed modern medicine and its practitioners, and spread misinformation about COVID-19 vaccines. Their petition also referred to instances where Ramdev lambasted modern medicine as a “stupid and bankrupt science” at a yoga session. The trigger was a series of Patanjali advertisements in Indian newspapers in July 2022 claiming that ayurvedic products could cure chronic conditions like diabetes, high blood pressure, heart diseases and autoimmune conditions. The Indian Medical Association’s petition alleged that such claims were in violation of India’s Drugs and Magic Remedies (Objectionable Advertisements) Act.
…The company’s public face – yoga guru Baba Ramdev – is a vocal supporter of India’s ruling party, the BJP, and Prime Minister Narendra Modi. Modi even inaugurated Patanjali’s ayurvedic research facility in 2017… Some scientists have accused their government of promoting these alternative medicines at the expense of modern medicine, partly as a way to glorify India’s culture and history. “One of the political ideas of this government is to glorify the Hindu tradition,” says Dhrubajyoti Mukherjee, president of the Breakthrough Science Society, an organization that promotes scientific thinking. “But in the name of our glorious past, the government is propagating obscure, unscientific ideas.”
… A few months after the outbreak of the COVID-19 pandemic in 2020, India’s health minister at the time, Harsh Vardhan participated in the company’s launch of pills, where Ramdev, the yoga guru, claimed the pills showed “100 percent favorable results” during clinical trials on patients. Despite experts flagging the lack of evidence, the company said it sold 2.5 million kits in six months, consisting of the tablets to ward off COVID-19 and bottled oils that would allegedly boost immunity. And the company is making an enormous amount of money: Its income was over $1.3 billion in the financial year 2021-22, with profits of $74 million before taxes.
Addressing the overall impact of misinformation about ayurvedic treatments, Dr. Jayesh Lele, vice president of the Indian Medical Association, says “Our worry is people are being misguided. We have got people who’ve left our treatment saying their kidneys will be able to function properly [using ayurvedic medicines] and ended up with renal failure. The same happened with patients suffering from hepatitis, who’ve got the wrong medicine and ended up with further problems. And if you say every day that modern medicine is bad, that is not acceptable.”
_______________
The sad thing, in my view, is that (as discussed previously) ayurvedic medicine has not just taken India for a ride:
- King Charles’ “Ayurvedic Centre of Excellence” turns out to be an embarrassing failure
- Dr Michael Dixon seems to support homeopathy as a treatment for cancer
- PRINCE CHARLES: the ‘immense value’ of alternative diagnostic methods
- Will the UK ‘ROYAL COLLEGE OF GENERAL PRACTITIONERS’ soon become a ‘ROYAL COLLEGE OF QUACKERY’?
And perhaps even more disappointing is the notion that, while in India they take action in order to prevent harm, I can see no such developments in the UK.
An alarming story of research fraud in the area of so-called alternative medicine (SCAM) is unfolding: Bharat B. Aggarwal, the Indian-American biochemist who worked at MD Anderson Cancer Center, focused his research on curcumin, a compound found in turmeric, and authored more than 125 Medline-listed articles about it. They reported that curcumin had therapeutic potential for a variety of diseases, including various cancers, Alzheimer’s disease and, more recently, COVID-19.
The last of these papers, entitled “Curcumin, inflammation, and neurological disorders: How are they linked?”, was publiched only a few months ago. Here is its abstract:
Background: Despite the extensive research in recent years, the current treatment modalities for neurological disorders are suboptimal. Curcumin, a polyphenol found in Curcuma genus, has been shown to mitigate the pathophysiology and clinical sequalae involved in neuroinflammation and neurodegenerative diseases.
Methods: We searched PubMed database for relevant publications on curcumin and its uses in treating neurological diseases. We also reviewed relevant clinical trials which appeared on searching PubMed database using ‘Curcumin and clinical trials’.
Results: This review details the pleiotropic immunomodulatory functions and neuroprotective properties of curcumin, its derivatives and formulations in various preclinical and clinical investigations. The effects of curcumin on neurodegenerative diseases such as Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), brain tumors, epilepsy, Huntington’s disorder (HD), ischemia, Parkinson’s disease (PD), multiple sclerosis (MS), and traumatic brain injury (TBI) with a major focus on associated signalling pathways have been thoroughly discussed.
Conclusion: This review demonstrates curcumin can suppress spinal neuroinflammation by modulating diverse astroglia mediated cascades, ensuring the treatment of neurological disorders.
The Anderson Cancer Center initially appeared to approve of Aggarwal’s work. However, in 2012, following concerns about image manipulation raised by pseudonymous sleuth Juuichi Jigen, MD Anderson Cancer Center launched a research fraud probe against Aggarwal which eventually led to 30 of Aggarwal’s articles being retracted. Moreover, PubPeer commenters have noted irregularities in many publications beyond the 30 that have already been retracted. Aggarwal thus retired from M.D. Anderson in 2015.
Curcumin doesn’t work well as a therapeutic agent for any disease – see, for instance, the summary from Nelson et al. 2017:
“[No] form of curcumin, or its closely related analogues, appears to possess the properties required for a good drug candidate (chemical stability, high water solubility, potent and selective target activity, high bioavailability, broad tissue distribution, stable metabolism, and low toxicity). The in vitro interference properties of curcumin do, however, offer many traps that can trick unprepared researchers into misinterpreting the results of their investigations.”
Despite curcumin’s apparent lack of therapeutic promise, the volume of research produced on curcumin grows each year. More than 2,000 studies involving the compound are now published annually. Many of these studies bear signs of fraud and involvement of paper mills. As of 2020, the United States National Institutes of Health (NIH) has spent more than 150 million USD funding projects related to curcumin.
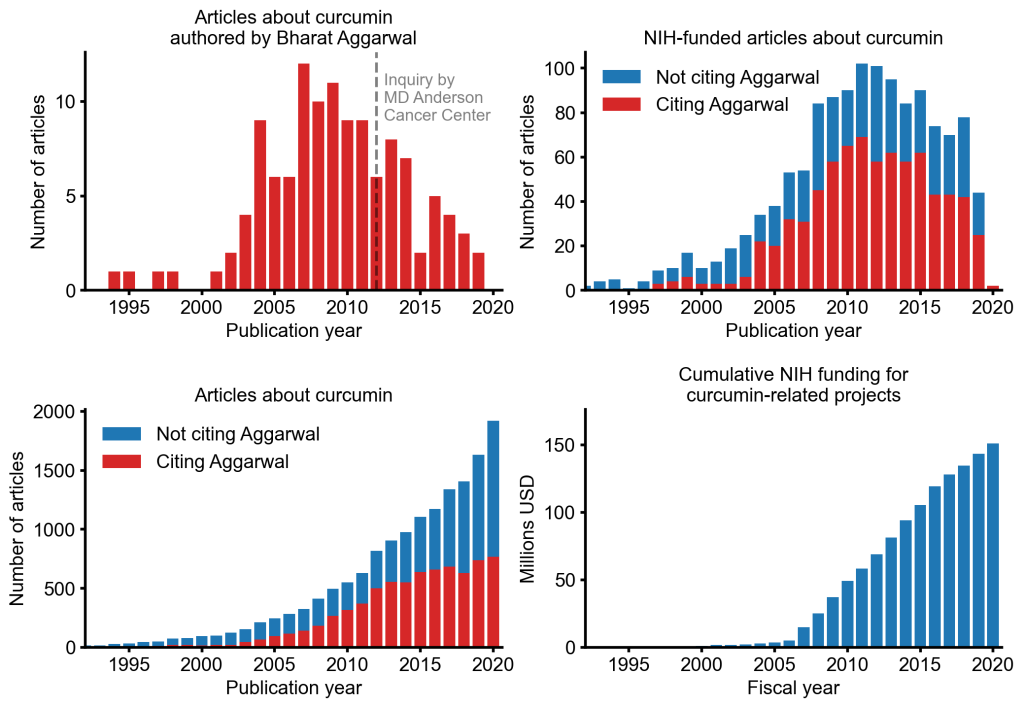
This proliferation of research has fueled curcumin’s popularity as a dietary supplement. It is estimated that the global market for curcumin as a supplement is around 30 million USD in 2020.
The damage done by this epic fraud is huge and far-reaching. Hundreds of millions of taxpayer dollars, countless hours spent toiling by junior scientists, thousands of laboratory animals sacrificed, thousands of cancer patients enrolled in clinical trials for ineffective treatments, and countless people who have eschewed effective cancer treatment in favor of curcumin, were encouraged by research steeped in lies.
It has been reported that King Charles’ charity, formerly the Prince’s Foundation, is compelled to return £110,000 to the Indian government. The funds were earmarked for an NHS alternative medicine clinic championed by Charles, which never materialised. The proposed clinic was aimed at integrating Indian traditional medicine into the UK’s healthcare system.
But why did the plan fail?
The answer is simple: the National Health Service (NHS) did not approve it.
The history of the UK ‘Ayurvedic Centre of Excellence’ goes back several years. Here is an excerpt of my book ‘CHARLES, THE ALTERNATIVE KING‘ where I discuss it as one of Charles’ many pipe dreams in the realm of so-called alternative medicine (SCAM):
In 2018, India’s prime minister Narendra Modi paid a visit to the Science Museum in London where he inspected the ‘5000 Years of Science and Innovation’ exhibition. The event was hosted by Charles and included the announcement of new ‘Ayurvedic Centres of Excellence’, allegedly a ‘first-of-its-kind’ global network for evidence-based research on yoga and Ayurveda. The first centre was said to open in 2018 in London. Funding was to come partly from the Indian government and partly from private donors. The central remit of the new initiative was reported to be researching the effects of Ayurvedic medicine.
Dr Michael Dixon (yes, you may have met him several times before, e.g. here, here, or here) commented: “This is going to be the first Ayurvedic centre of excellence in the UK. We will be providing, on the NHS, patients with yoga, with demonstrations and education on healthy eating, Ayurvedic diets, and massage including reflexology and Indian head massage. And all this will be subject to a research project led by Westminster University, to find out whether the English population will take to yoga and these sorts of treatments. Whether they will be helped by it and finally whether it will reduce the call on NHS resources leading to less GP consultations, hospital admissions and operations.”
On its website, the College of Medicine and Integrated Health announced that a memorandum of understanding with India’s Ministry of Ayurveda, Yoga and Naturopathy, Unani, Siddha and Homoeopathy (AYUSH) had been signed “to create centres of excellence in the UK … Dr Michael Dixon agreed the joint venture to provide the UK centres, which will offer and research traditional Indian medicine… The Indian government will match private UK donations to fund the AYUSH centres in the UK”. In November 2019, the following press release by the president of India offered more details:
The Prince of Wales called on the President of India, Shri Ram Nath Kovind, at Rashtrapati Bhavan today (November 13, 2019).
Welcoming the Prince to India, the President congratulated him on his election as the head of the Commonwealth. He said that India considers the Commonwealth as an important grouping that voices the concerns of a large number of countries, including the Small Island Developing States.
The President said that India and the United Kingdom are natural partners bound by historical ties and shared values of democracy, rule of law and respect for multi-cultural society. As the world’s pre-eminent democracies, our two countries have much to contribute together to effectively address the many challenges faced by the world today.
The Prince planted a Champa sapling – plant native to the subcontinent which has several uses in Ayurveda – in the Herbal Garden of Rashtrapati Bhavan. He was taken around the garden and shown different plants that have medicinal properties. The Prince showed a keen interest in India’s alternative model of healthcare.
The President thanked the Prince of Wales for his support for Ayurveda research. The Prince of Wales Charitable Foundation and the All India Institute of Ayurveda signed an MOU during the visit of Prime Minister Narendra Modi to the UK in April 2018. Under the MOU, the All India Institute of Ayurveda and the College of Medicine, UK will be conducting clinical research on Depression, Anxiety and Fibromyalgia. They will also be undertaking training programme for the development of Standard Operating Protocol on “AYURYOGA” for UK Health professionals.
_________________________
END OF EXCERPT
Charles’ initiative, encompassing Ayurveda, yoga, naturopathy, and homeopathy, was intended to be a landmark project, with the Indian government contributing £110,000 to the King’s Foundation for its implementation. However, the NHS, responsible for St Charles Hospital, never endorsed the project. Despite initial talks, the proposed collaboration did not progress, and the clinic failed to materialise. According to the west London clinical commissioning group (CCG), which oversaw the hospital at the time, there was no official involvement, and discussions ceased in 2020.
Under charity law, funds designated for a specific project cannot be diverted without donor permission and regulatory approval. The King’s Foundation has acknowledged the need to return the remaining budget to the Indian government but has not disclosed when this decision was made or why the funds were not promptly returned.
The initiative faced opposition from the NHS, as a year before the clinic’s launch, NHS England’s CEO Simon Stevens had issued guidance discouraging the prescription of homeopathy and herbal remedies, citing their limited efficacy and misuse of NHS funds.
Despite the failed project, connections between key figures persist. Dr Michael Dixon played a significant role in finalising agreements with the Indian government. The King’s Foundation defended its actions, stating that due to the Covid-19 pandemic, the project shifted online, resulting in reduced costs. They claim to have contacted the Indian government for the return of unused funds, emphasising that the money remains in a restricted account.
As the controversy unfolds, questions arise about the intersections between alternative medicine advocacy, royal endorsements, and international collaborations within the context of public healthcare.
An article in the Daily Mail reported that the original plan proposed that Ayush treatments would be provided to patients, who would be referred by local GPs, at St Charles Hospital in Kensington. Isaac Mathai, who runs Soukya, a homeopathic yoga retreat in Bangalore which Charles and Camilla have visited, was an adviser to the project at St Charles Hospital.
The Indian government made a payment from the budget of the Ayush Ministry, which Mr Modi has used as a tool of diplomacy to promote Indian medicine and culture worldwide, to the King’s Foundation. It was proposed the charity would use its expertise to help set up the clinic. But the NHS at no point agreed to the plans.
A spokesman of the west London clinical commissioning group (CCG), which administered St Charles Hospital at the time, said: ‘Provision of homeopathy and herbal treatments were not considered as part of the project by the CCG. The aim of the project was to test the use of yoga and massage to support the overall health and wellbeing of patients with long-term conditions.’ A King’s Foundation spokesman added that the initial intention had been to deliver Indian traditional medicine at St Charles Hospital.
During the coronavirus disease 2019 pandemic, Ayurvedic herbal supplements and homeopathic remedies were promoted as immune boosters (IBs) and disease-preventive agents. This happened in most parts of the world but nowhere more intensely than in India.
The present study examined the clinical outcomes among patients with chronic liver disease who presented with complications of portal hypertension or liver dysfunction temporally associated with the use of IBs in the absence of other competing causes. This Indian single-center retrospective observational cohort study included patients with chronic liver disease admitted for the evaluation and management of jaundice, ascites, or hepatic encephalopathy temporally associated with the consumption of IBs and followed up for 180 days. Chemical analysis was performed on the retrieved IBs.
From April 2020 to May 2021, 1022 patients with cirrhosis were screened, and 178 (19.8%) were found to have consumed complementary and alternative medicines. Nineteen patients with cirrhosis (10.7%), jaundice, ascites, hepatic encephalopathy, or their combination related to IBs use were included. The patients were predominantly male (89.5%). At admission, 14 (73.75%) patients had jaundice, 9 (47.4%) had ascites, 2 (10.5%) presented with acute kidney injury, and 1 (5.3%) had overt encephalopathy. Eight patients (42.1%) died at the end of the follow-up period. Hepatic necrosis and portal-based neutrophilic inflammation were the predominant features of liver biopsies.
Ten samples of IBs, including locally made ashwagandha powder, giloy juice, Indian gooseberry extracts, pure giloy tablets, multiherbal immune-boosting powder, other multiherbal tablets, and the homeopathic remedy, Arsenicum album 30C, were retrieved from our study patients. Samples were analyzed for potential hepatotoxic prescription drugs, known hepatotoxic adulterants, pesticides, and insecticides, which were not present in any of the samples. Detectable levels of arsenic (40%), lead (60%), and mercury (60%) were found in the samples analyzed. A host of other plant-derived compounds, industrial solvents, chemicals, and anticoagulants was identified using GC–MS/MS. These include glycosides, terpenoids, phytosteroids, and sterols, such as sitosterol, lupeol, trilinolein, hydroxy menthol, methoxyphenol, butyl alcohol, and coumaran derivatives.
The authors concluded that Ayurvedic and Homeopathic supplements sold as IBs potentially cause the worsening of preexisting liver disease. Responsible dissemination of scientifically validated, evidence-based medical health information from regulatory bodies and media may help ameliorate this modifiable liver health burden.
The authors comment that Ayurvedic herbal supplements and homeopathic remedies sold as IBs, potentially induce idiosyncratic liver injury in patients with preexisting liver disease. Using such untested advertised products can lead to the worsening of CLD in the form of liver failure or portal hypertension events, which are associated with a high risk of mortality compared to those with severe AH-related liver decompensation in the absence of timely liver transplantation. Severe mixed portal inflammation and varying levels of hepatic necrosis are common findings on liver histopathology in IB-related liver injury. Health regulatory authorities and print and visual media must ensure the dissemination of responsible and factual scientific evidence-based information on herbal and homeopathic “immune boosters” and health supplements to the public, specifically to the at-risk patient population.
It has been reported that King Charles refused to pay Prince Andrew’s £ 32,000-a-year bill for his personal healing guru. The Duke of York has allegedly submitted the claim to the Privy Purse as a royal expense having sought the help of a yoga teacher.
However, the claim has reportedly been denied by the King, who is said to have told Andrew the bill will need to be covered using his own money. It comes after sources claimed Andrew has been using the Indian yogi for a number of years for chanting, massages, and holistic therapy in the privacy of his mansion. The healer has reportedly enjoyed month-long stays at a time at the £30 million Royal Lodge in Windsor.
Previously, the Queen seems to have passed the claims. But now Charles is in control. A source said: “While the Queen was always happy to indulge her son over the years, Charles is far less inclined to fund such indulgences, particularly in an era of a cost-of-living crisis. “Families are struggling and would rightly baulk at the idea of tens of thousands paid to an Indian guru to provide holistic treatment to a non-working royal living in his grace and favour mansion. This time the King saw the bill for the healer submitted by Andrew to the Privy Purse and thought his brother was having a laugh.”
________________________
Poor Andrew!
How is he going to cope without his guru?
Will he be able to recover from the mysterious condition that prevents him to sweat?
Will his ego take another blow?
How will he be able to afford even the most basic holistic wellness?
How can Charles – who knows only too well about its benefits – be so cruel to his own brother?
Should I start a collection so that Andrew can pay for his most basic needs?
____________________________
Yes, these are the nagging questions and deep concerns that keep me awake at night!
PS
I have just been asked if, by any chance, the yoga teacher is a 16-year-old female. I have to admit that I cannot answer this question.
Gut microbiota can influence health through the microbiota–gut–brain axis. Meditation can positively impact the regulation of an individual’s physical and mental health. However, few studies have investigated fecal microbiota following long-term (several years) deep meditation. Therefore, this study tested the hypothesis that long-term meditation may regulate gut microbiota homeostasis and, in turn, affect physical and mental health.
To examine the intestinal flora, 16S rRNA gene sequencing was performed on fecal samples of 56 Tibetan Buddhist monks and neighboring residents. Based on the sequencing data, linear discriminant analysis effect size (LEfSe) was employed to identify differential intestinal microbial communities between the two groups. Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) analysis was used to predict the function of fecal microbiota. In addition, we evaluated biochemical indices in the plasma.
The α-diversity indices of the meditation and control groups differed significantly. At the genus level, Prevotella and Bacteroides were significantly enriched in the meditation group. According to the LEfSe analysis, two beneficial bacterial genera (Megamonas and Faecalibacterium) were significantly enriched in the meditation group. The functional predictive analysis further showed that several pathways—including glycan biosynthesis, metabolism, and lipopolysaccharide biosynthesis—were significantly enriched in the meditation group. Moreover, plasma levels of clinical risk factors were significantly decreased in the meditation group, including total cholesterol and apolipoprotein B.
The Chinese authors concluded that the intestinal microbiota composition was significantly altered in Buddhist monks practicing long-term meditation compared with that in locally recruited control subjects. Bacteria enriched in the meditation group at the genus level had a positive effect on human physical and mental health. This altered intestinal microbiota composition could reduce the risk of anxiety and depression and improve immune function in the body. The biochemical marker profile indicates that meditation may reduce the risk of cardiovascular diseases in psychosomatic medicine. These results suggest that long-term deep meditation may have a beneficial effect on gut microbiota, enabling the body to maintain an optimal state of health. This study provides new clues regarding the role of long-term deep meditation in regulating human intestinal flora, which may play a positive role in psychosomatic conditions and well-being.
This study is being mentioned on the BBC new-bulletins today – so I thought I have a look at it and check how solid it is. The most obvious question to ask is whether the researchers compared comparable samples.
The investigators collected a total of 128 samples. Subsequently, samples whose subjects had taken antibiotics and yogurt or samples of poor quality were excluded, resulting in 56 eligible samples. To achieve mind training, Tibetan Buddhist monks performed meditation practices of Samatha and Vipassana for at least 2 hours a day for 3–30 years (mean (SD) 18.94 (7.56) years). Samatha is the Buddhist practice of calm abiding, which steadies and concentrates the mind by resting the individual’s attention on a single object or mantra. Vipassana is an insightful meditation practice that enables one to enquire into the true nature of all phenomena. Hardly any information about the controls was provided.
This means that dozens of factors other than meditation could very easily be responsible for the observed differences; nutrition and lifestyle factors are obvious prime candidates. The fact that the authors fail to even discuss these possibilities and more than once imply a causal link between meditation and the observed outcomes is more than a little irritating, in my view. In fact, it amounts to very poor science.
I am dismayed that a respected journal published such an obviously flawed study without a critical comment and that the UK media lapped it up so naively.
Quackery is rife in India. On this blog, I have occasionally reported on this situation, e.g.:
- The new ‘WHO Global Centre for Traditional Medicine’ in India
- Mucormycosis (black fungus): is the Indian AYUSH ministry trying to decimate the population?
- Homeopathy, COVID, India and Prince Charles: not a good mixture!
- Has homeopathy caused the dramatic decline of COVID-19 cases in India?
- Homeopathy research from India is far from trustworthy, and today I can show you why
- Brazil and India collaborate in the promotion of quackery
- Taking the piss again? The story of urine therapy in India
- The intriguing case of homeopathy in India
- Prince Charles’ advocacy of quackery is by no means harmless
- Patient Dies After Homeopath Gives Wrong Injection
- Herbal remedies are good for you … except for the ones that injure your liver
- The ‘AYUSH COVID-19 Helpline’: have they gone bonkers?
Now the Chief Justice of India (CJI) NV Ramana has pointed out that legislation needs to be brought in to save people “from falling prey to fraudulent practices in the name of treatment”. Speaking at the inaugural National Academy of Medical Sciences on ‘Law and Medicine’, the CJI said: “Quackery is the biggest disease affecting India” and that hospitals are “being run like companies, where profit-making is more important than service to society”. The CJI added, “another side of lack of accessible healthcare is giving space to quacks. Quackery begins where awareness ends. Where there is room for myths, there is room for quackery”. He continued, “Owing to the financial and time constraints, a huge majority of the Indian population approaches these untrained and uncertified doctors. Lack of awareness and knowledge, misplaced belief, and sheer inaccessibility have massive ramifications on the health of the country, particularly the rural and underprivileged Indian … The need of the hour is to bring in legislation to save people from falling prey to fraudulent practices in the name of treatment … Private hospitals are being opened at an exponential rate. This is not necessarily a bad thing, but there is a glaring need for balance. We are seeing hospitals being run like companies, where profit-making is more important than service to society.”
I am sure the CJI is correct; India does have a quackery problem. If nothing else, the fact that one website lists a total of 746 Alternative Medicine Colleges in India, leaves little doubt about it.
Camilla spent ten days at the end of October in a sophisticated meditation and fitness center in southern India. Life has recently been hectic for the Queen Consort: at 75, she has been in a non-stop succession of various ceremonies for the funeral of Elizabeth II, always one step behind her husband, not to mention her new status as sovereign… Enough to block her chakras in no time.
She came to the resort with her bodyguards and a handful of friends and was able to take advantage of the tailor-made treatments concocted for her by the master of the house, Dr Issac Mathai, who created this high-end holistic centre on a dozen hectares of scented gardens near Bangalore. The program includes massages, herbal steam baths, yoga, naturopathy, homeopathy, meditation, and Ayurvedic treatments to “cleanse, de-stress, soothe and revitalize the mind, body and soul”, as the establishment’s website states.
Guests are required to follow an individualized, meat-free diet, with organic food from the resort’s vegetable gardens, based on lots of salads or soups – Camilla is said to be a fan of sweet corn soup with spinach. Cigarettes and mobile phones are not allowed, although it is assumed that Camilla must have some privileges due to her status… and the basic rate for the suites, which starts at $950 a night – the price of the rooms varies between $260 and $760, the rate including a consultation with the doctors.
Charles and Camilla have been fans of the Soukya Centre in India for a decade. The place corresponds in every way to their deep-rooted convictions about health. Like her husband, Camilla is a follower of organic food, she also practices yoga and treats her face with creams made from nettle and bee venom. For his part, Charles has long been an advocate of alternative medicine, homeopathy, acupuncture, aromatherapy, and also hypnosis… He even set up a foundation to support complementary medicine by lobbying the British health service to include it in complementary therapies for certain patients, which caused an uproar among the pundits of traditional medicine.
________________________
If you suspected I was (yet again) sarcastic about the royal couple, you are mistaken. The text above is only my (slightly shortened) translation of an article published in the French magazine LE POINT (even the title is theirs). I found the article amusing and interesting; so, I looked up the Indian health center. Here are some of the things I found:
The 1st impression is that they are not shy about promotion calling themselves THE WORLD’S BEST AYURVEDA TREATMENT CENTER. The doctor in charge was once a ‘Consultant Physician’ at the Hale Clinic in London, where he treated a number of high-profile people. As his professional background, he offers this:
M.D. (Homeopathy); Hahnemann Post-Graduate Institute of Homeopathy, London M.R.C.H, London; Chinese Pulse Diagnosis and Acupuncture, WHO Institute of Traditional Chinese Medicine, Nanjing, China; Trained (Mind-Body Medicine Programme) at Harvard Medical School, USA
The approach of the center is described as follows:
The fundamental principle underlying Holistic Treatment is that the natural defense and immune system of an individual when strengthened, has the potential to heal and prevent diseases. In the age of super-specialisation where human beings are often viewed as a conglomeration of organs, it is crucial to understand ourselves as multi-dimensional beings with a body, mind and spirit. These interconnected dimensions need to be in perfect harmony to ensure real well-being.
And about homeopathy, they claim this:
Homeopathy originated in 1796 in Germany, and was discovered by Dr. Samuel Hahnemann, a German scientist. Homeopathy is popular today as a non-intrusive, holistic system of medicine. Instead of different medicines for different parts of the body, one single constitutional remedy is prescribed. As a system of medicine, Homeopathy is highly scientific, safe, logical and an extremely effective method of healing. For over 200 years people have used Homeopathy to maintain their good health, and also to treat and cure a wide range of illnesses like allergies, metabolic disorders, atopic dermatitis, Rheumatoid arthritis, Auto-immune disorders.
At this stage, I felt I had seen enough. Yes, you are right, we did not learn a lot from this little exploration. No, hold on! We did learn that homeopathy is highly scientific, safe, logical, and extremely effective!
The question, however, is should we believe it?


