regulation
If you google ‘chiropractic’ you might get the impression that an unusual number of US chiros are outright perverts. Here are four current cases that I found instantly without any in-depth seraching:
A “Christian chiropractor” is facing several criminal charges after at least eight former clients have accused him of rape and sexual assault. Roc Byrd, 61, of Danville, who worked as a chiropractor for Cornerstone Chiropractic in Avon, is facing one felony count of rape, five felony counts of sexual battery and four misdemeanor counts of battery. He is accused of raping a client, touching clients inappropriately over and under their clothes during appointments without consent and pressing his genitals up against multiple patients. Byrd identified himself as a practicing Christian and reportedly began each of his chiropractic appointments by praying with clients.
A Warren chiropractor faces significant legal problems and a criminal investigation into how he allegedly treated one of his patients. Officials say he sells himself as one of only a handful of Michigan “Chiropractic Neurologists,” but the former patient claims he is a sexual predator and offers video as apparent proof. The case involves Dr. John Pispidikis of the Spinal Recovery Center. The complaint was filed Friday (April 19) morning, which surprised the doctor. The patient involved remains unnamed in the civil court documents, but she claims the doctor groped her during a physical exam last February. But she had no proof, so she claims she went out and got some.
The Oklahoma Board of Chiropractic Examiners (OBCE) has ordered the Back Stop’s only chiropractor, Mark Kimble to surrender his license by next Monday after several sexual impropriety allegations against him have surfaced. Oklahoma Board of Chiropractic Examiners confirmed with KFOR that there are seven alleged victims of Kimble’s who have come forward.
The Los Angeles County Sheriff’s Department is looking for possible victims of a chiropractor accused of sexual assault. Richard Carnow, 65, was arrested by authorities on March 13 on four felony counts of sexual battery. Carnow is a chiropractor in San Dimas in the San Gabriel Valley, the Sheriff’s Department says. He’s accused of sexually assaulting multiple adult women between June 2023 and August 2023. Officials did not say if his alleged victims were current or former patients. Investigators say the nature of the alleged crimes have led them to believe there may be additional victims and they are asking for the public’s help to find them.
Yes, I know, these are (according to chiros’ assurances) regrettable, isolated cases – nothing to worry about!
But perhaps these assertions are wrong and there is a problem after all?
I am reminded of my post from 2021; let me refresh your memory:
Two chiropractors conducted a retrospective review of publicly available data from the California Board of Chiropractic Examiners. Their aim was to determine categories of offense, experience, and gender of disciplined doctors of chiropractic (DC) in California and compare them with disciplined medical physicians in California. The DC disciplinary categories, in descending order, were
- fraud (44%),
- sexual boundary issues (22%),
- other offences (13%),
- abuse of alcohol or drugs (10%),
- negligence or incompetence (6%),
- poor supervision (2%),
- mental impairment (.3%).
The authors concluded that the professions differ in the major reasons for disciplinary actions. Two thirds (67%) of the doctors of chiropractic were disciplined for fraud and sexual boundary issues, compared with 59% for negligence and substance misuse for medical physicians. Additional study in each profession may reveal methods to identify causes and possible intervention for those who are at high risk.
The abstract of the paper does not provide comparisons to with the medical profession. Here they are; relative to doctors, chiropractors are:
- 2 x more likely to be involved in malpractice,
- 9 x more likely to commit fraud,
- 2 x more likely to transgress sexual boundaries.
________________________
Could it be, I askmyself, that there is something deeply wrong with the chiropractic profession? Could it perhaps be that chiro schools do not have a good hand when it comes to student recruitment? Could it be that chiro schools teach too little medical ethics, or none at all?
An interesting and fully referenced (205 references) article caught my attention; it seems highly relevant to the discussions we are having on this blog. Let me show you the abstract:
Medical misinformation has always existed, but it has recently become more frequent due to the development of the internet and social media. Medical misinformation can cover a wide variety of topics, and studies show that some groups are more likely to be affected by medical misinformation than others, like those with less trust in health care, less health literacy, and a more positive attitude toward alternative medicines. Aspects of the internet, like echo chambers and algorithms, have contributed to the rise of medical misinformation, along with belief in anecdotal evidence and alternative remedies that are not backed by science. Some personal beliefs and a lack of media literacy skills are also contributing to medical misinformation. Medical misinformation causes higher rates of death and negative health outcomes, a lack of trust in medical professionals, and more racism and hate crimes. One possible way to combat the spread of misinformation is education surrounding media literacy. Still, there are gaps in this practice that must be addressed like a lack of high-quality research about different educational programs.
The author also offers the following key points:
- Medical misinformation is becoming an urgent issue for United States citizens—leading to increased deaths,
a lack of trust in health professionals, and hate crimes and racism. - Although this is a worldwide issue, the United States has the second highest rate of misinformation of any
country, behind India. - One piece of misinformation during the COVID-19 pandemic stated that highly concentrated alcohol could
disinfect the body and kill the virus. Studies show that 800 people died, 5,876 were hospitalized, and 60
became completely blind from drinking methanol, thinking it would cure coronavirus. - Studies estimate that only 14% of the United States population has proficient health literacy, which makes it difficult to recognize medical misinformation.
- Media literacy education is being pursued in order to combat the spread of misinformation, but more research is needed in order to understand the long-term effects of this education and what programs are best.
__________________
I would like to stress, as indeeed the author does as well, that medical misinformation is a phenomenon that is by no means confined to the US. Like most information, misinformation has become a global issue. Its dangers cannot be under-estimated. My blog offers an abundance of reports where misinformation in the realm of so-called alternative medicine (SCAM) has caused harm and even death. The author advocates media literacy as a remedy for the problem. I would argue that even more important would be to teach CRITICAL THINKING, a task that has to start at school and must continue well into adult life.
This conclusion is so very obvious that it begs an important question: WHY HAS IT NOT BEEN DONE YEARS AGO? The answer, I fear, is simple: for reasons that are self-evident, governments have little interst in the public being able to think critically. On the contrary, governments across the world foremost want to be re-elected, and critical thinking would be a major obstacle to this aim.
It has been reported that 5 people who took a Japanese health supplement have died and more than 100 have been hospitalized as of Friday, a week after a pharmaceutical company issued a recall of the products, officials said. Osaka-based Kobayashi Pharmaceutical Co. came under fire for not going public quickly with problems known internally as early as January. Yet the first public announcement came only on 22 March. Company officials said 114 people were being treated in hospitals after taking products — including Benikoji Choleste Help meant to lower cholesterol — that contain an ingredient called benikoji, a red species of mold. Some people developed kidney problems after taking the supplements, but the exact cause was still under investigation in cooperation with government laboratories, according to the manufacturer.
“We apologize deeply,” President Akihiro Kobayashi told reporters last Friday, bowing for a long time to emphasize the apology alongside three other top company officials. He expressed remorse to those who have died and have been taken ill and to their families. He also apologized for the troubles caused to the entire health food industry and the medical profession, adding that the company was working to prevent further damage and improve crisis management.
The company’s products have been recalled — as have dozens of other products that contain benikoji, including miso paste, crackers, and a vinegar dressing. Japan’s health ministry put up a list on its official site of all the recalled products, including some that use benikoji for food coloring. The ministry warned the deaths could keep growing. The supplements could be bought at drug stores without a prescription from a doctor, and some may have been purchased or exported before the recall, including by tourists who may not be aware of the health risks.
Kobayashi Pharmaceutical had been selling benikoji products for years, with a million packages sold over the past 3 fiscal years, but a problem crept up with the supplements produced in 2023. Kobayashi Pharmaceutical said it produced 18.5 tons of benikoji last year. Some analysts blame the recent deregulation initiatives, which simplified and sped up approval for health products to spur economic growth.
________________________
Anouther source reported that Japanese authorities on Saturday raided a drug factory after a pharmaceutical company reported at least five deaths and 114 hospitalizations possibly linked to a health supplement. About a dozen Japanese health officials walked into the Osaka plant of the Kobayashi Pharmaceutical Co., as seen in footage of the raid widely telecasted on Japanese news. The health supplement in question is a pink pill called Benikoji Choleste Help. It is said to help lower cholesterol levels. A key ingredient is benikoji, a type of red mold. The company has said it knows little about the cause of the sickness, which can include kidney failure. It is currently investigating the effects in cooperation with Japan’s government.
___________________________
More recent reports update the figure of affected individuals: Japanese dietary supplements at the center of an expanding health scare have now been linked to at least 157 hospitalizations, a health ministry official said Tuesday.The figure reflects an increase from the 114 hospitalization cases that Kobayashi Pharmaceutical said on Friday were linked to its products containing red yeast rice, or beni kōji.
A Kobayashi Pharmaceutical spokeswoman confirmed the latest hospitalization cases without elaborating further.
Benikoji is widely sold and used; not just in Japan. It comes under a range of different names:
- red yeast rice,
- red fermented rice,
- red kojic rice,
- red koji rice,
- anka,
- angkak,
- Ben Cao Gang Mu.
It is a bright reddish purple fermented rice which acquires its color from being cultivated with the mold Monascus purpureus. Red yeast rice is used as food and as a medicine in Asian cultures, such as Kampo and TCM.
It contains lovastatin which, of course, became patented and is marketed as the prescription drug, Mevacor. Red yeast rice went on to become a non-prescription dietary supplement in the United States and other countries. In 1998, the U.S. FDA banned a dietary supplement containing red yeast rice extract, stating that red yeast rice products containing monacolin K are identical to a prescription drug, and thus subject to regulation as a drug.
An article about chiropractic caught my attention. Let me show you its final section which, I think, is relevant to what we often discuss on this blog:
If chiropractic treatment is unscientific, then why do I feel better? Because lots of things alleviate pain. Massage, analgesia and heat – but also a provider who listens, empathises and bothers to examine a patient. Then there is the placebo effect. For centuries, doctors have recognised that different interventions with unclear pathways result in clinical improvement. Among the benefits patients attributed to placebo 100 years ago: “I sleep better; my appetite is improved; my breathing is better; I can walk further without pain in my chest; my nerves are steadier.” Nothing has changed. Pain is a universal assignment; no one has a monopoly on its relief.
The chiropractic industry owes its existence to a ghost. Its founder, David Palmer, wrote in his memoir The Chiropractor that the principles of spinal manipulation were passed on to him during a séance by a doctor who had been dead for half a century. Before this, Palmer was a “magnetic healer”.
Today, chiropractors preside over a multibillion-dollar regulated industry that draws patients for various reasons. Some can’t find or afford a doctor, feel dismissed, or worse, mistreated. Others mistrust the medical establishment and big pharma. Still others want natural healing. But none of these reasons justifies conflating a chiropractor with a doctor. The conflation feels especially hazardous in an environment of health illiteracy, where the mere title of doctor confers upon its bearer strong legitimacy.
Chiropractors don’t have the same training as doctors. They cannot issue prescriptions or order advanced imaging. They do not undergo lifelong peer review or open themselves to monthly morbidity audits.
I know that doctors could do with a dose of humility, but I can’t find any evidence (or the need) for the assertion on one website that chiropractors are “academic overachievers”. Or the ambit claim that most health professionals have no idea how complicated the brain is, but chiropractors do.
Forget doctors, patients deserve more respect.
My friend’s back feels better for now. When it flares, I wonder if she will seek my advice – and I am prepared to hear no. Everyone is entitled to see a chiropractor. But no patient should visit a chiropractor thinking that they are seeing a doctor.
______________________
I would put it more bluntly:
- chiropractors are poorly trained; in particular, they do not learn to question their own, often ridiculous beliefs;
- they are poorly regulated; in the UK, the GCC seems to protect the chiros rather than the public;
- chiropractors regularly disregard essential rules of medical ethics, e.g. informed consent;
- many try to mislead us by pretending they are physicians;
- their hallmark intervention, spinal manipulation, can cause considerable harm;
- it generates hardly any demonstrable benefit for any condition;
- chiropractors also cause considerable harm, e.g. by interfering with real medicine, e.g. vaccinations;
- thus, in general, chiropractors do more harm than good;
- yes, everyone is entitled to see a chiropractor, but before they do, reliable information should be mandatory.
The Amercian Medical Association (AMA) recently published a lengthy article on naturopathy in the US. Here are some excerpts:
There are three types of health professionals who offer naturopathic treatment:
- Naturopathic doctors. These nonphysicians graduate from a four-year, professional-level program at an accredited naturopathic medical school, earning either the doctor of naturopathy (ND) degree or the doctor of naturopathic medicine (NMD) degree.
- Traditional naturopaths, who have obtained education through some combination of a mentorship program with another professional or at an alternative clinic, distance-learning program or classroom schooling on natural health, or other holistic studies.
- Other health professionals such as chiropractors, massage therapists, dentists, nurses, nutritionists, or physicians who practice under a professional license but include some naturopathic methods in their practice and who may have studied on their own or taken courses on naturopathic methods.
At least 24 states and the District of Columbia regulate the practice of naturopathy. In order to be licensed, naturopaths in these states must earn an ND or NMD from an accredited naturopathic program and pass the Naturopathic Physicians Licensing Exam. Three states—Florida, South Carolina and Tennessee—prohibit the practice of naturopathy. In states that neither license nor prohibit the practice of naturopathy, traditional naturopaths and NDs alike may practice without being subject to state regulation.
Postgraduate training is neither common nor required of graduates of naturopathic schools, except in Utah … less than 10% of naturopaths participate in an approved residency, and such residencies last only a year and lack a high degree of standardization.
… naturopaths are required to get at least 1,200 hours of direct patient contact, physicians get 12,000–16,000 hours of clinical training…
ND programs emphasize naturopathic principes—for example, the healing power of nature—and naturopathic therapeutics such as botanical medicine, homeopoathy and hydrotherapy. Coursework in naturopathic therapeutics is combined with, and taught alongside, coursework in sciences. But there are no specifications around the number of hours required in each area … naturopathic students may lack exposure to key clinical scenarios in the course of their training … naturopathic students’ clinical experience is typically gained through outpatient health care clinics, as naturopathic medical schools typically do not have significant hospital affiliation. This means there is no guarantee that a naturopathic student completing a clinical rotation will see patients who are actually sick or hospitalized, and they may not be exposed to infants, children, adolescents or the elderly. It has been said that naturopaths tend to treat the “worried well.”
… Naturopaths claim they are trained as primary care providers and, as such, are educated and trained to diagnose, manage and treat many conditions, including bloodstream infections, heart disease and autoimmune disorders. Yet their education and training falls several years and thousands of hours short of what physicians get.
…The AMA believes it is the responsibility of policymakers to ensure that naturopaths’ claims that they can treat a broad range of conditions are backed by facts—facts that include the specific education and training necessary to ensure patient safety.
________________
The AMA is clearly cautious here. A less polite statement might simply stress that naturopaths are taught a lot of nonsense which they later tend to administer to their unsuspecting patients. On this blog, we have repeatedly discussed the danger naturopaths present to public health in the US and elsewhere, e.g.:
- How reliable are the claims made by naturopathic influencers?
- Naturopath jailed for selling fraudulent vaccination documents
- Naturopath fined for misdiagnosing and treating a rectal tumor for hemorrhoids
- Naturopaths are ‘not bound by science,’ lawyer argues
- Vaccination rates of Canadian healthcare professionals: those of chiropractors and naturopaths are at the lowest
- Is veterinary naturopathy animal abuse?
- Naturopathic ‘cancer specialist’ using coffee enemas found guilty
- Patients consulting chiropractors, homeopaths, or naturopaths are less likely to agree to the flu jab
- A naturopath responsible for the death of two cancer patients was sentenced to two years
- A naturopath in court after two of his cancer patients died
- Many naturopaths, homeopaths, and chiropractors are a risk to public health
- Naturopath treats autism with fecal transplants
- A naturopath promoting fake news about COVID vaccinations
- Naturopathy (according to the WNF) = quackery steeped in obsolete fantasies
- Canadian naturopaths may no longer call themselves ‘medically trained’
- Naturopaths’ counselling against vaccinations could be criminally negligent
- Naturopathy for cancer … claims that have the potential to be lethal
- Severe liver injury due to naturopaths’ prescription of Epsom salt
- Naturopaths should not treat children
- Some naturopaths are clearly a danger to public health
- Death of a child through naturopathy
Claims that naturopaths are a viable alternative to evidence-based medicine are wrong, irresponsible and dangerous. Regulators must be reminded that they have the duty to protect the public from charlatans and should therefore ensure that no false therapeutic or diagnostic claims can be made by naturopaths.
I usually take ‘market reports’ with a pinch of salt. Having said that, this document makes some rather interesting predictions:
The size of the market for so-called alternative medicine (SCAM) is projected to expand from USD 147.7 billion in 2023 to approximately USD 1489.4 billion by the year 2033. This projection indicates a remarkable Compound Annual Growth Rate (CAGR) of 26% over the forecast period.
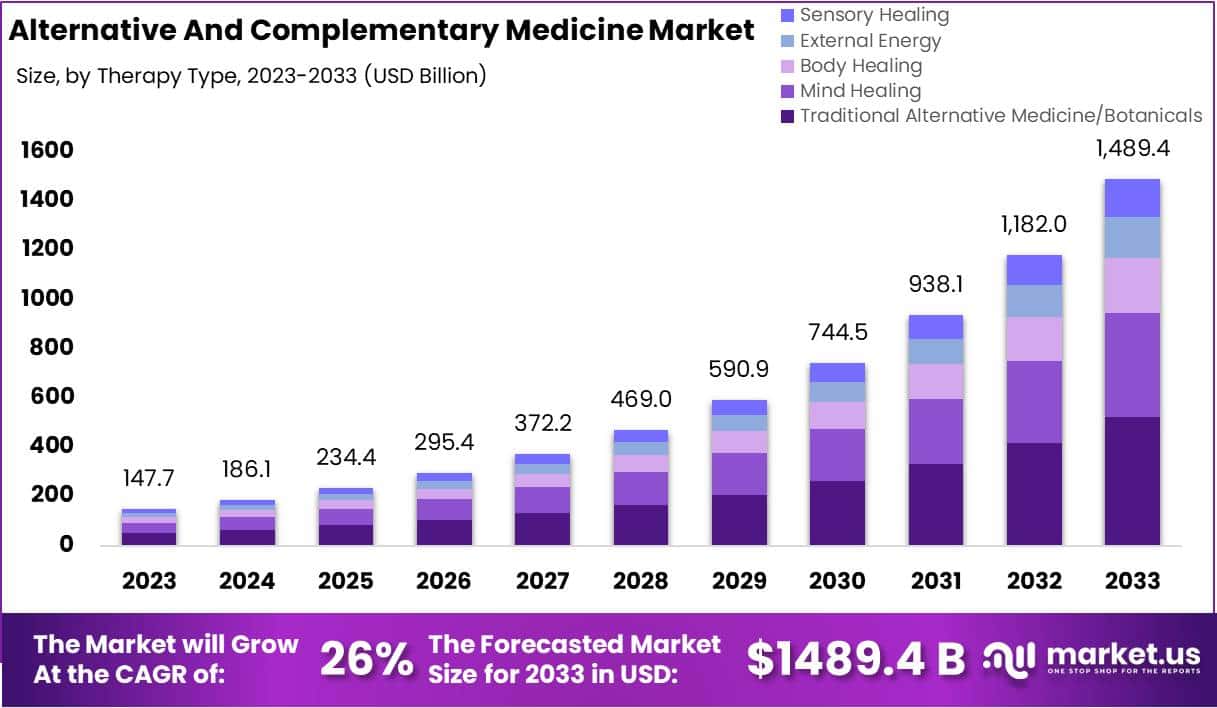
The market for SCAM is experiencing significant growth, fueled by increasing consumer interest in natural and holistic health solutions. This trend reflects a broader shift in societal attitudes towards health and wellness, emphasizing preventive care and natural health practices.
The market’s dynamics are influenced by various factors, including consumer preferences, regulatory standards, and evolving perceptions of health and wellness. As the popularity of these alternative therapies grows, it is crucial for individuals to consult with healthcare professionals to ensure that these non-conventional approaches are safely and effectively incorporated into their overall health regimen. The increasing acceptance of SCAM underscores a collective move towards more personalized and holistic healthcare solutions, resonating with today’s health-conscious consumers.
In 2023, Traditional Alternative Medicine/Botanicals led the market, capturing a 35.2% share, which reflects a strong consumer inclination towards these treatments. Dietary Supplements were prominent in the market, securing a 25.1% share in 2023, which underscores the high consumer demand for nutritional aids. Direct Sales were the most favored distribution channel, accounting for 43.2% of the market share in 2023, which indicates their significant impact on guiding consumer purchases. Pain Management was the predominant application area, holding a 24.9% market share in 2023, propelled by the growing acknowledgment of non-pharmacological treatment options. Adults represented a substantial portion of the market, making up 62.33% in 2023, signifying a marked preference for SCAM therapies within this age group. Europe stood out as the market leader, claiming a 42.6% share in 2023, a position supported by widespread acceptance, governmental backing, and an increasing elderly population. The regions of North America and Asia-Pacific are highlighted as areas with potential, signaling opportunities for market expansion beyond the European stronghold in the upcoming years.
Leading Market Players Are:
- Columbia Nutritional
- Nordic Nutraceuticals
- Ramamani Iyengar Memorial Yoga Institute
- The Healing Company Ltd.
- John Schumacher Unity Woods Yoga Centre
- Sheng Chang Pharmaceutical Company
- Pure encapsulations LLC.
- Herb Pharm
- AYUSH Ayurvedic Pte Ltd.
Recent developments:
- In December 2023, Adoratherapy launched the Alkemie Chakra Healing Line, an aromatherapy range aimed at harmonizing the seven chakras.
- Coworth Park introduced the Hebridean Sound Treatment in October 2023, merging traditional Hebridean sounds with guided meditation to offer a novel, restorative wellness experience.
- The World Health Organization released draft guidelines in September 2023 for the safe, effective application of traditional medicines.
- Telehealth services, expanding significantly in August 2023, have broadened the reach of SCAM, enhancing patient access to these treatments.
Traditional herbal medicine (THM) is frequently used in pediatric populations. This is perticularly true in many low-income countries. Yet THM has been associated with a range of adverse events, including liver toxicity, renal failure, and allergic reactions. Despite these concerns, its impact on multi-organ dysfunction syndrome (MODS) risk has so far not been thoroughly investigated.
This study aimed to investigate the incidence and predictors of MODS in a pediatric intensive care unit (PICU) in Ethiopia, with a focus on the association between THM use and the risk of MODS. It was designed as a single-center prospective cohort study conducted at a PICU in the university of Gondar Comprehensive Specialized hospital, Northwest Ethiopia. The researchers enrolled eligible patients aged one month to 18 years admitted to the PICU during the study period. Data on demographic characteristics, medical history, clinical and laboratory data, and outcome measures using standard case record forms, physical examination, and patient document reviews. The predictors of MODS were assessed using Cox proportional hazards models, with a focus on the association between traditional herbal medicine use and the risk of MODS.
A total of 310 patients were included in the final analysis, with a median age of 48 months and a male-to-female ratio of 1.5:1. The proportion and incidence of MODS were 30.96% (95% CI:25.8, 36.6) and 7.71(95% CI: 6.10, 9.40) per 100-person-day observation respectively. Renal failure (17.74%), neurologic failure (15.16%), and heart failure (14.52%) were the leading organ failures identified. Nearly one-third of patients (32.9%) died in the PICU, of which 59.8% had MODS. The rate of mortality was higher in patients with MODS than in those without. The Cox proportional hazards model identified renal disease (AHR = 6.32 (95%CI: 3.17,12.61)), intake of traditional herbal medication (AHR = 2.45, 95% CI:1.29,4.65), modified Pediatric Index of Mortality 2 (mPIM 2) score (AHR = 1.54 (95% CI: 1.38,1.71), and critical illness diagnoses (AHR = 2.68 (95% CI: 1.77,4.07)) as predictors of MODS.
The authors concluded that the incidence of MODS was high. Renal disease, THM use, mPIM 2 scores, and critical illness diagnoses were independent predictors of MODS. A more than twofold increase in the risk of MODS was seen in patients who used TMH. Healthcare providers should be aware of risks associated with THM, and educate caregivers about the potential harms of these products. Future studies with larger sample sizes and more comprehensive outcome measures are needed.
I do fully agree with the authors about the high usage of herbal and other so-called alternative medicines by children. We have shown that, in the UK the average one-year prevalence rate was 34% and the average lifetime prevalence was 42%. We have furthermore shown that the evidence base for these treatments in children is weak, even more so than for general populations. Finally, we can confirm that adverse effects are far from rare and often serious.
It is therefore high time, I think, that national regulators do more to protect children from SCAM practitioners who are at best uncritical about their treatments and at worse outright dangerous.
The French ‘National Assembly’ has yesterday adopted a major law aimed at reinforcing the prevention and combat against sectarian aberrations in France. This marks a significant step forward in strengthening the protection of citizens against abuse and manipulation by charlatans, gurus and other sectarian movements.
This bill, the result of particularly fruitful work and debate in both chambers, reflects the Government’s commitment to meeting the expectations of the victims of these sectarian movements.
Some of the key measures voted through by parliamentarians include:
- The enshrinement in law of the powers of MIVILUDES (Interministerial Mission of Vigilance and Combat against Sectarian Aberrations);
- The reinforcement of the penal response with the creation of the offence of placing or maintaining in a state of psychological or physical subjection;
- The creation of an offence of incitement to abandon or refrain from treatment, or to adopt practices which clearly expose the person concerned to a serious health risk;
- Support for victims, with the extension of the categories of associations that can bring civil action;
- Information for the judiciary, with the introduction of an “amicus curiae” role for certain government departments in legal cases relating to cults.
Despite sometimes heated debates, particularly around article 4, fuelled by the opinion of the Conseil d’Etat, the adoption of this law by the National Assembly bears witness to a shared desire to protect the rights and freedoms of individuals while providing better protection for our fellow citizens against sectarian aberrations.
This bill is part of a multi-annual national strategy for 2023-2027 resulting from the conference on sectarian aberrations held in spring 2023. It is a major step towards strengthening the penal arsenal and protecting victims.
_______________
Sabrina Agresti-Roubache, Secretary of State for Citizenship and Urban Affairs, commented:
“Long-awaited by victim support associations, this text aims to strengthen our legal arsenal in the fight against sectarian aberrations. I’m delighted that all the articles have been adopted, particularly Article 4, which creates an offence of incitement to abandon or abstain from treatment. There have been some passionate debates in the Chamber, but I’d like to reiterate the basis of this bill: the State is not fighting against beliefs, opinions or religions, but against all forms of sectarian aberrations, these dangerous behaviors which represent a threat to our social cohesion and put lives at risk.”
_______________
Obviously, we shall have to see how the new law will be applied. But, in any case, it is an important step into the right direction and could put an end to much of so-called alternative medicine that endangers the health of French consumers.
Other nations should consicer following the Franch example.
We have often asked whether the General Chiropractic Council (GCC) is fit for purpose. A recent case bought before the Professional Conduct Committee (PCC) of the GCC provides further food for thought.
The male chiropractor in question admitted to the PCC that:
- he had requested the younger female patient remove her clothing to her underwear for the purposes of examination;
- he then treated the area near her vagina and groin with a vibrating tool;
- that he also treated the area around her breasts.
After the appointment, which the patient had originally booked for a problem with her neck, the patient reflected on the treatment and eventually complained about the chiropractor to the GCC. The PCC considered the case and did not find unprofessional conduct in the actions and conduct of the chiropractor. His the diagnosis and treatment were both found to be clinically justified.
According to the GCC, the lesson from this case is that the complaint to the GCC may have been avoided if the chiropractor had been more alert to the need to ensure he communicated effectively so that the patient was clear as to why the intimate areas were being treated and, on that basis, given informed consent. Patients often feel vulnerable before, during and after treatment; and this effect is magnified when the patient is unclothed, new to chiropractic treatment or the work of a particular chiropractor, or they are being treated in an intimate area. Chiropractors can reduce this feeling of vulnerability by offering a chaperone and gown (and recording a note of the patient’s response) as well as taking the time to ensure you have fully explained the procedure to them and obtained informed consent. Standard D4 of the GCC Code states registrants must “Consider the need, during assessments and care, for another person to be present to act as a chaperone; particularly if the assessment or care might be considered intimate or where the patient is a child or a vulnerable adult.”
Excuse me?
I find this unbelievably gross and grossly unbelievable!
It begs, I think, the following questions:
- What condition requires treatment with a ‘vibrating tool’ near the vagina (I assume they mean vulva)?
- What condition requires treatment with a ‘vibrating tool’ around the breasts?
- Is there any reliable evidence?
- Was informed consent obtained?
- What precisely did it entail?
About 15 years ago, I was an expert witness in a very similar UK case. The defendant was sent to prison for two years. The GCC is really not fit for purpose. It seems to consistently defend chiropractors rather than do its duty and defend their patients.
My advice to the above-mentioned patient is not to bother with the evidently useless GCC but to initiale criminal proceedings.
The so-called ‘Miracle Mineral Solution’ (MMS) – bleach for you and me – is a SCAM that keeps on giving. On this blog, we have featured MMS several times before, e.g.:
- Selling bleach as ‘miracle’ cure (MMS): Father and three sons are going to prison
- Selling bleach solution as ‘miracle’ cure? No, it’s a dangerous ‘snake oil’!
- Miracle Mineral Supplement (MMS): accidental ingestion by an infant
- Beware of the ‘Bleach Boys’ – hydrogen peroxide and chlorine dioxide
Now,it has been reported that a New Zealand anti-vaxxer has been jailed for selling more than $100,000 worth of an industrial bleach as a “miracle” cure for Covid-19. Roger Blake, who describes himself as a “human man”, was sentenced to just over 10 months’ imprisonment after being found guilty at trial of 29 charges in the Hamilton District Court.
Blake advertised and sold MMS products, claiming it could treat, prevent and cure coronavirus. However, New Zealand’s Ministry of Health had not approved the product, and detailed that when ingested became chlorine dioxide – a bleach commonly used for water treatment, bleaching textiles and paper.
 The court heard Blake had marketed the product as a cure in New Zealand from the start of the pandemic between December 2019 and December 2020. Medsafe, the health ministry’s safety authority, said Blake’s company had sales of NZ$160,000 in that period – with sales spiking in March when the country was placed in lockdown.
The court heard Blake had marketed the product as a cure in New Zealand from the start of the pandemic between December 2019 and December 2020. Medsafe, the health ministry’s safety authority, said Blake’s company had sales of NZ$160,000 in that period – with sales spiking in March when the country was placed in lockdown.
Judge Brett Crowley said Blake’s behaviour had been “utterly disgraceful”. He added that Blake had “seized upon the tragedy” of the pandemic for financial gain. Before selling MMS as a “cure” for the coronavirus, Blake had marketed the product as a preventive of other diseases and illnesses such as cancer, Alzheimer’s, diabetes and HIV.
Medsafe prosecuted him under the Medicines Act, with compliance manager Derek Fitzgerald saying the “fake cure” Blake spruiked presented a “significant public health risk”. “He targeted the vulnerable, preyed on public fears and exposed people to harm”, he said. “This decision sends a strong message that people who engage in selling so called ‘miracle cures’ will be held to account and face fines or imprisonment.”
The website which sold MMS in New Zealand was registered to US-based Mark Grenon, who set up the “Genesis II Church of Health and Healing”. As reported previously, Grenon and his three sons were jailed in October for several years in the US for selling more than US$1m of the product. Michael Homer, an assistant US lawyer who prosecuted the case, said at the time the family targeted people suffering from life-threatening illnesses. The Grenons poisoned thousands of people with their bogus miracle cure, which was nothing more than industrial bleach,” he said.
Medsafe warns: “Drinking MMS is the same as drinking bleach and can cause dangerous side effects, including severe vomiting, diarrhoea, and life-threatening low blood pressure. We strongly encourage people to only go to trusted sources, such as your doctor, to get reliable information”.
Medsafe received three reports of people requiring hospitalizations after drinking MMS. “His conduct presented a significant risk to public health, and that is why Medsafe acted. His actions were in stark contrast to the requirements of the Medicines Act 1981, which is public welfare legislation designed to protect the public” said Mr Fitzgerald.
