herbal medicine
This study aimed to evaluate the effect of Traditional Chinese Medicine (TCM) on patients with gastric cancer following surgery and adjuvant chemotherapy in Taiwan. The cohort sampling data set was obtained from the Registry of Catastrophic Illness Patient Database, a research database of patients with severe illnesses from the National Health Insurance Research Database, Taiwan. Patients who had received a new diagnosis of gastric cancer and had undergone surgery were enrolled. the researchers matched TCM users and nonusers at a ratio of 1 : 3 based on the propensity score, and TCM users were also grouped into short-term and long-term users.
The number of TCM users and nonusers was 1701 and 5103 after applying the propensity score at a ratio of 1 : 3. Short-term users and long-term TCM users were independently associated with a decreased risk of death with HRs of 0.59 (95% confidence interval (CI), 0.55-0.65) and 0.41 (95% CI, 0.36-0.47), respectively, compared with TCM nonusers. The researchers also obtained similar results when they adjusted for covariates in the main model, as well as each of the additional listed covariates. They also observed similar HR trends in short-term users and long-term TCM users among men and women aged <65 years and ≥65 years. The most commonly prescribed single herb and herbal formula in our cohort were Hwang-Chyi (Radix Hedysari; 11.8%) and Xiang-Sha-Liu-Jun-Zi-Tang (15.5%), respectively.

The authors concluded that TCM use was associated with higher survival in patients with gastric cancer after surgery and adjuvant chemotherapy. TCM could be used as a complementary and alternative therapy in patients with gastric cancer after surgery and adjuvant chemotherapy.
This is an interesting study which seems well-done – except for one fatal mistake: even in the title, the authors imply a causal relationship between TCM and survival. Their conclusion has two sentences; the first one speaks correctly of an association. The second, however, not only implies causality but goes much further in suggesting that TCM should be used to prolong the life of patients. Yet, there are, of course, dozens of factors that could interfere with the findings or be the true cause of the observed outcome.
Anyone with a minimum of critical thinking ability should know that CORRELATION IS NOT CAUSATION; sadly, the authors of this study seem to be the exception.
On Twitter, the hype had begun even before its text was available. Priti Gandhi, for instance, tweeted:
Yet another feather in India’s cap!! 1st evidence-based, CoPP-WHO GMP certified medicine for Covid-19 released today. Congratulations to @yogrishiramdev ji, @Ach_Balkrishna ji & the team of scientists at Patanjali Research Institute. Your efforts have been successful!! #Ayurveda
So, what is it all about? This study included 100 patients and was designed to evaluate the impact of traditional Indian Ayurvedic treatment on asymptomatic patients with COVID-19 infection. It is a placebo-controlled randomized double-blind pilot clinical trial that was conducted at the Department of Medicine in the National Institute of Medical Sciences and Research, Jaipur, India.
- 1 g of Giloy Ghanvati (Tinospora cordifolia)
- 2 g of Swasari Ras (traditional herbo-mineral formulation)
- 0.5 g of Ashwagandha (Withania somnifera)
- 0.5 g of Tulsi Ghanvati (Ocimum sanctum)
The treatment was given orally to the patients in the treatment group twice per day for 7 days. Medicines were given in the form of tablets and each tablet weighed 500 mg. While Swasari Ras was administered in powdered form, 30 min before breakfasts and dinners, rest were scheduled for 30 min post-meals. Patients in the treatment group also received 4 drops of Anu taila (traditional nasal drop) in each nostril every day 1 h before breakfast. Patients in the placebo group received identical-looking tablets and drops, post-randomization, and double-blinded assortments. 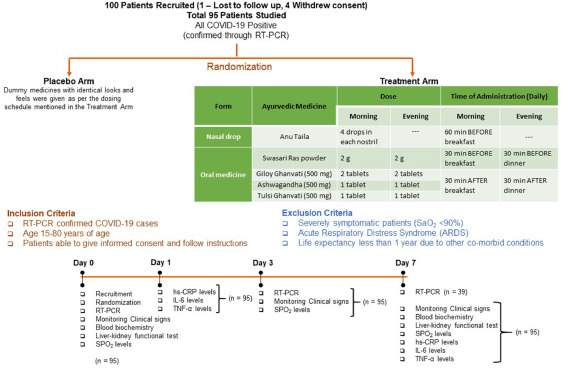 The RT-qPCR test was used for the detection of viral load in the nasopharyngeal and oropharyngeal swab samples of study participants during the study. Chemiluminescent immunometric assay was used to quantify serum levels of interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), and high sensitivity C-reactive protein (hs-CRP) on day 1 and day 7 of the study. Patient testing negative for SARS-CoV-2 in the RT-PCR analysis was the primary outcome of this study.
The RT-qPCR test was used for the detection of viral load in the nasopharyngeal and oropharyngeal swab samples of study participants during the study. Chemiluminescent immunometric assay was used to quantify serum levels of interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), and high sensitivity C-reactive protein (hs-CRP) on day 1 and day 7 of the study. Patient testing negative for SARS-CoV-2 in the RT-PCR analysis was the primary outcome of this study.
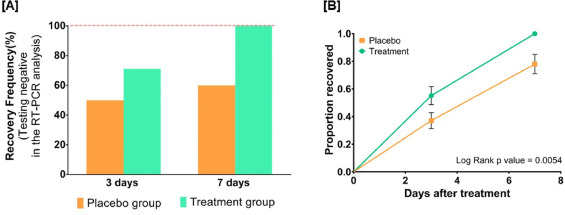
By day three, 71.1 % and 50.0 % of patients recovered in the treatment and placebo groups, respectively. The treatment group witnessed 100 % recovery by day 7, while it was 60.0 % in the placebo group. Average fold changes in serum levels of hs-CRP, IL-6, and TNF-α in the treatment group were respectively, 12.4, 2.5 and 20 times lesser than those in the placebo group at day 7. There was a 40 % absolute reduction in the risk of delayed recovery from infection in the treatment group.
The authors concluded that Ayurvedic treatment can expedite virological clearance, help in faster recovery and concomitantly reduce the risk of viral dissemination. Reduced inflammation markers suggested less severity of SARS-CoV-2 infection in the treatment group. Moreover, there was no adverse effect observed to be associated with this treatment.
I have the following concerns or questions about this trial:
- Why do the authors call it a pilot study? A pilot study is merely for testing the feasibility of a trial design and is not meant to yield definitive efficacy results.
- The authors state that the patients were asymptomatic yet in the discussion they claim they were asymptomatic or mildly symptomatic.
- Some of the effect sizes reported here are extraordinary and seem almost too good to be true.
- The claim of no adverse effect is implausible; even placebos would cause perceived adverse effects in a percentage of patients.
- If the study is solid and withstands the scrutiny of the raw data, it is of huge relevance for public health. So, why did the authors publish it in PHYTOMEDICINE, a relatively minor and little-known journal?
An article in The Economic Times’ reported this:
Patanjali Ayurved released what it called the first “evidence-based” medicine for Covid-19 on Friday. It claimed it has been “recognised by the WHO (World Health Organization) as an ayurvedic medicine for corona”.
Patanjali promoter, yoga guru Baba Ramdev, released a scientific research paper in this regard at the launch, presided over by Union health minister Harsh Vardhan and transport minister Nitin Gadkari.
The Ayurveda products maker said it has received a certification from the Ayush ministry. “Coronil has received the Certificate of Pharmaceutical Product (CoPP) from the Ayush section of Central Drugs Standard Control Organisation (CDSCO) as per the WHO certification scheme,” it said in a statement.
Under the CoPP, Coronil can be exported to 158 countries, the company said, adding that based on the presented data, the ministry has recognised Coronil as medicine for “supporting measure in Covid-19”.
Am I the only one who fears that something is not entirely kosher about the study? (This is an honest question, and I would be pleased to receive answers from my readers)
“Today, scientists note that the glycyrrhizic acid contained in this plant prevents the development of a new coronavirus, which the whole world is fighting against. Moreover, even a small concentration of an aqueous extract of licorice root has a neutralizing effect.”

These are the words of President Gurbanguly Berdymukhamedov of Turkmenistan. The plant he referred to is licorice. With is the promotion of a herbal solution for the pandemic, he is in good company:
- Thailand’s health ministry approved the use of Andrographis Paniculata, commonly known as green chiretta, to treat patients who are in the early stages of a Covid-19 infection.
- The health authorities of Tamil Nadu distributed herbal medicine to the general public as a preventive measure against Coronavirus disease.
- Madagascar claims to have a cure for Covid-19, the herbal tea named Covid-Organics has the plant artemisia as an ingredient.
- China has been using TCM alongside conventional treatment methods to treat Covid-19 patients. Some of the herbal formulations used in the treatment are:
- Jinhua Qinggan Granule
- Sheganmahuang decoction
- Lianhuaqingwen capsule
- Maxingshigan decoction
- Xuebijing Injection
- Indonesia is testing two herbal medicines: Cordyceps militaris, a fungus common in the Himalayas, and a herbal formulation comprising Ginger, gripeweed, Ngai camphor, and Andrographis paniculata.
And what about some evidence? In 2020, Medline listed 302 articles on herbal medicine for COVID-19. Here I selected just 10 of them to give you a flavor:
COVID-19 is the most recently discovered coronavirus infectious disease and leads to pandemic all over the world. The clinical continuum of COVID-19 varies from mild illness with non-specific signs and symptoms of acute respiratory disease to extreme respiratory pneumonia and septic shock. It can transmit from animal to human in the form of touch, through the air, water, utensils, fomite and feco-oral route blood. The pathogenesis and clinical features of COVID-19 be the same as the clinical manifestation associated epidemic Fever. In Unani medicine, various herbal drugs are described under the caption of epidemic disease. Great Unani scholar also Avicenna (980-1037 AD) recommended that during epidemic condition movement should be restricted, self-isolation, fumigation around the habitant with perfumed herbs (Ood, Kafoor, Sumbuluttib, Saad Kofi, Loban, etc.), and use of appropriate antidotes (Tiryaqe Wabai) and vinegar (Sirka) as prophylaxis. Herbal approach is based on single (Unnab-Ziziphus jujuba, Sapistan-Cordia myxa, Bahidana-Cydonia oblonga, Khatmi-Althea officinalis, Khubazi-Malva sylvestris, Zafran-Crocus sativus, Sibr-Aloe barbedensis, Murmuki-Commiphora myrrha, Darchini-Cinnamomum zeylanicum, Qaranfal-Syzygium aromaticum, Rihan-Oscimum sanctum, Habtus Sauda-Nigella sativa, Aslus Sus-Glycyrrhiza glabra, Maghze Amaltas-Cassia fistula and Adusa-Adhatoda vasica) and compound drugs (Habbe Bukhar, Sharbat Khaksi, Sharbat Zanjabeel, Naqu Nazla, Majoon Chobchini, Jawrish Jalinus and Khamira Marvareed) most of them are claimed for anti-viral, anti-pyretic, blood purifier, cardioprotective and expectorant activities. Traditionally most of the herbal practitioners are using it.
According to the World Health Organization (WHO), viral diseases continue to rise, and pose a significant public health problem. Novel coronavirus disease (COVID-19) is an infectious disease caused by SARS-CoV-2. The pathogenesis and clinical manifestations of COVID-19 is close to Amraz-e-Wabai (epidemic diseases) which was described by Hippocrates, Galen, Aristotle, Razes, Haly Abbas, Avicenna, Jurjani etc. Presently, there is no specific or challenging treatment available for COVID-19. Renowned Unani Scholars recommended during epidemic situation to stay at home, and fumigate the shelters with aromatics herbs like Ood kham (Aquilaria agallocha Roxb.), Kundur (Boswellia serrata Roxb), Kafoor (Cinnamomum camphora L.), Sandal (Santalum album L), Hing (Ferula foetida L.) etc. Use of specific Unani formulations are claimed effective for the management of such epidemic or pandemic situation like antidotes (Tiryaqe Wabai, Tiryaqe Arba, Tiryaqe Azam, Gile Armani), Herbal Decoction (Joshandah), along with Sharbate Khaksi, Habbe Bukhar, Sharbate Zanjabeel, Khamira Marwareed, Jawarish Jalinus, and Sirka (vinegar). Such drugs are claimed for use as antioxidant, immunomodulatory, cardiotonic, and general tonic actions. The study enumerates the literature regarding management of epidemics in Unani medicine and attempts to look the same in the perspective of COVID-19 prevention and management.
Unani system of medicine is based on the humoral theory postulated by Hippocrates, according to him the state of body health and disease are regulated by qualitative and quantitative equilibrium of four humours. Amraz-e-Waba is an umbrella term which is used in Unani medicine for all types of epidemics (smallpox, measles, plague, Hameer Saifi, influenza, Nipaha, Ebola, Zika, and 2019 novel coronavirus, etc.) mostly fatal in nature. The coronavirus disease 2019 (COVID-19) is a severe acute respiratory infection, and the pathogenesis and clinical features resemble with those of Nazla-e-Wabaiya (influenza) and Zatul Riya (pneumonia) which were well described many years ago in Unani text such as high-grade fever, headache, nausea and vomiting, running nose, dry cough, respiratory distress, alternate and small pulse, asthenia, foul smell from breath, insomnia, frothy stool, syncope, coldness in both upper and lower extremities, etc. The World Health Organization declared COVID-19 as a global emergency pandemic. Unani scholars like Hippocrates (370-460 BC), Galen (130-200 AD), Rhazes (865-925 AD), and Avicenna (980-1037 AD) had described four etiological factors for Amraz-e-Waba viz., change in quality of air, water, Earth, and celestial bodies, accordingly mentioned various preventive measures to be adopted during epidemics such as restriction of movement, isolation or “quarantena”, and fumigation with loban (Styrax benzoin W. G. Craib ex Hartwich.), sandalwood (Santalum album L.), Zafran (Crocus sativus L.), myrtle (Myrtus communis L.), and roses (Rosa damascena Mill.) and use of vinegar (sirka) and antidotes (Tiryaq) as prophylaxis, and avoiding consumption of milk, oil, sweet, meat, and alcohol. This review focuses and elaborates on the concept, prevention, and probable management of COVID-19 in the light of Amraz-e-Waba.
Background: Current recommendations for the self-management of SARS-Cov-2 disease (COVID-19) include self-isolation, rest, hydration, and the use of NSAID in case of high fever only. It is expected that many patients will add other symptomatic/adjuvant treatments, such as herbal medicines.
Aims: To provide a benefits/risks assessment of selected herbal medicines traditionally indicated for “respiratory diseases” within the current frame of the COVID-19 pandemic as an adjuvant treatment.
Method: The plant selection was primarily based on species listed by the WHO and EMA, but some other herbal remedies were considered due to their widespread use in respiratory conditions. Preclinical and clinical data on their efficacy and safety were collected from authoritative sources. The target population were adults with early and mild flu symptoms without underlying conditions. These were evaluated according to a modified PrOACT-URL method with paracetamol, ibuprofen, and codeine as reference drugs. The benefits/risks balance of the treatments was classified as positive, promising, negative, and unknown.
Results: A total of 39 herbal medicines were identified as very likely to appeal to the COVID-19 patient. According to our method, the benefits/risks assessment of the herbal medicines was found to be positive in 5 cases (Althaea officinalis, Commiphora molmol, Glycyrrhiza glabra, Hedera helix, and Sambucus nigra), promising in 12 cases (Allium sativum, Andrographis paniculata, Echinacea angustifolia, Echinacea purpurea, Eucalyptus globulus essential oil, Justicia pectoralis, Magnolia officinalis, Mikania glomerata, Pelargonium sidoides, Pimpinella anisum, Salix sp, Zingiber officinale), and unknown for the rest. On the same grounds, only ibuprofen resulted promising, but we could not find compelling evidence to endorse the use of paracetamol and/or codeine.
Conclusions: Our work suggests that several herbal medicines have safety margins superior to those of reference drugs and enough levels of evidence to start a clinical discussion about their potential use as adjuvants in the treatment of early/mild common flu in otherwise healthy adults within the context of COVID-19. While these herbal medicines will not cure or prevent the flu, they may both improve general patient well-being and offer them an opportunity to personalize the therapeutic approaches.
Recently, the novel life-threatening coronavirus infection (COVID-19) was reported at the end of 2019 in Wuhan, China, and spread throughout the world in little time. The effective antiviral activities of natural products have been proved in different studies. In this review, regarding the effective herbal treatments on other coronavirus infections, promising natural products for COVID-19 treatment are suggested. An extensive search in Google Scholar, Science Direct, PubMed, ISI, and Scopus was done with search words include coronavirus, COVID-19, SARS, MERS, natural product, herb, plant, and extract. The consumption of herbal medicine such as Allium sativum, Camellia sinensis, Zingiber officinale, Nigella sativa, Echinacea spp. Hypericum perforatum, and Glycyrrhiza glabra, Scutellaria baicalensis can improve the immune response. It seems that different types of terpenoids have promising effects in viral replication inhibition and could be introduced for future studies. Additionally, some alkaloid structures such as homoharringtonine, lycorine, and emetine have strong anti-coronavirus effects. Natural products can inhibit different coronavirus targets such as S protein (emodin, baicalin) and viral enzymes replication such as 3CLpro (Iguesterin), PLpro (Cryptotanshinone), helicase (Silvestrol), and RdRp (Sotetsuflavone). Based on previous studies, natural products can be introduced as preventive and therapeutic agents in the fight against coronavirus.
Background: The aim of the present review is to provide basic knowledge about the treatment of Coronavirus via medicinal plants. Coronavirus (COVID-19, SARS-CoV, and MERS-CoV) as a viral pneumonia causative agent, infects thousands of people in China and worldwide. There is currently no specific medicine or vaccine available and it is considered a threat to develop effective novel drug or anti-coronavirus vaccine treatment. However, natural compounds to treat coronaviruses are the most alternative and complementary therapies due to their diverse range of biological and therapeutic properties.
Methods: We performed an open-ended, English restricted search of Scopus database, Web of Science, and Pubmed for all available literature from Jan-March, 2020, using terms related to phytochemical compounds, medicinal plants and coronavirus.
Results: The view on anti-coronavirus (anti-CoV) activity in the plant derived phytochemicals and medicinal plants give the strong base to develop a novel treatment of corona virus activity. Various phytochemicals and medicinal plant extracts have been revised and considered to be the potential anti-CoV agents for effective control and future drug development. We discuss some important plants (Scutellaria baicalensis, Psorothamnus arborescens, Glycyrrhiza radix, Glycyrrhiza uralensis , Lycoris radiate, Phyllanthus emblica, Camellia sinensis, Hyptis atrorubens Poit, Fraxinus sieboldiana, Erigeron breviscapus, Citri Reticulatae Pericarpium, Amaranthus tricolor, Phaseolus vulgaris, Rheum palmatum, Curcuma longa and Myrica cerifera) emerged to have broad spectrum antiviral activity.
Conclusion: Nigella sativa has potent anti-SARS-CoV activity and it might be useful souce for developing novel antiviral therapies for coronaviruses.
COVID-19 has been declared a pandemic by WHO on March 11, 2020. No specific treatment and vaccine with documented safety and efficacy for the disease have been established. Hence it is of utmost importance to identify more therapeutics such as Chinese medicine formulae to meet the urgent need. Qing Fei Pai Du Tang (QFPDT), a Chinese medicine formula consisting of 21 herbs from five classical formulae has been reported to be efficacious on COVID-19 in 10 provinces in mainland China. QFPDT could prevent the progression from mild cases and shorten the average duration of symptoms and hospital stay. It has been recommended in the 6th and 7th versions of Clinical Practice Guideline on COVID-19 in China. The basic scientific studies, supported by network pharmacology, on the possible therapeutic targets of QFPDT and its constituent herbs including Ephedra sinica, Bupleurum chinense, Pogostemon cablin, Cinnamomum cassia, Scutellaria baicalensis were reviewed. The anti-oxidation, immuno-modulation and antiviral mechanisms through different pathways were collated. Two clusters of actions identified were cytokine storm prevention and angiotensin converting enzyme 2 (ACE2) receptor binding regulation. The multi-target mechanisms of QFPDT for treating viral infection in general and COVID-19 in particular were validated. While large scale clinical studies on QFPDT are being conducted in China, one should use real world data for exploration of integrative treatment with inclusion of pharmacokinetic, pharmacodynamic and herb-drug interaction studies.
In December 2019, a novel coronavirus SARS-CoV-2, causing the disease COVID-19, spread from Wuhan throughout China and has infected people over 200 countries. Thus far, more than 3,400,000 cases and 240,000 deaths have occurred worldwide, and the coronavirus pandemic continues to grip the globe. While numbers of cases in China have been steadying, the number of infections outside China is increasing at a worrying pace. We face an urgent need to control the spread of the COVID-19 epidemic, which is currently expanding to a global pandemic. Efforts have focused on testing antiviral drugs and vaccines, but there is currently no treatment specifically approved. Traditional Chinese medicine (TCM) is grounded in empirical observations and the Chinese people use TCM to overcome these sorts of plagues many times in thousands of years of history. Currently, the Chinese National Health Commission recommended a TCM prescription of Qing-Fei-Pai-Du-Tang (QFPDT) in the latest version of the “Diagnosis and Treatment guidelines of COVID-19” which has been reported to provide reliable effects for COVID-19. While doubts about TCM still exist today, this review paper will describe the rationalities that QFPDT is likely to bring a safe and effective treatment of COVID-19.
The fight against the novel coronavirus pneumonia (namely COVID-19) that seriously harms human health is a common task for all mankind. Currently, development of drugs against the novel coronavirus (namely SARS-CoV-2) is quite urgent. Chinese medical workers and scientific researchers have found some drugs to play potential therapeutic effects on COVID-19 at the cellular level or in preliminary clinical trials. However, more fundamental studies and large sample clinical trials need to be done to ensure the efficacy and safety of these drugs. The adoption of these drugs without further testing must be careful. The relevant articles, news, and government reports published on the official and Preprint websites, PubMed and China National Knowledge Infrastructure (CNKI) databases from December 2019 to April 2020 were searched and manually filtered. The general pharmacological characteristics, indications, adverse reactions, general usage, and especially current status of the treatment of COVID-19 of those potentially effective drugs, including chemical drugs, traditional Chinese medicines (TCMs), and biological products in China were summarized in this review to guide reasonable medication and the development of specific drugs for the treatment of COVID-19.
Objective: To analysis the medication characteristics of the prescriptions issued via open channel by the National and Provincial Health Committee and the State Administration of Traditional Chinese Medicine in treating coronavirus disease 2019 (COVID-19).
Methods: We collected the data of traditional Chinese medicine related to treatment plans published by the National and Provincial Health Committee and the State Administration of Traditional Chinese Medicine from the start of COVID-19 outbreak to February 19, 2020. The frequency analysis, cluster analysis and association analysis were performed.
Results: The study collected 4 national and 34 regional prevention and treatment plans, 578 items, 84 traditional Chinese formulations, 60 Chinese patent medicines, and 230 Chinese herbs. The high frequently used herbs were Liquorice, Scutellariabaicalensis, Semen armeniacaeamarae, and Gypsum. The commonly used traditional formulations included Maxing Shigan decoction, Yin Qiao powder, and Xuanbai Chengqi decoction. The Chinese patent drugs included Angong Niuhuang pill, Xuebijing injection, and Lianhua Qingwen capsule. The most common paired medications were Ephedra and Semen armeniacaeamarae, Fructusforsythiae and Liquorice. Two core combinations and one novel formula were discovered in the study.
Conclusions: Yin Qiao powder and Huopo Xialing decoction are the basic formulations for Weifen syndrome of COVID-19. In addition, Maxing Shigan decoction, Liang Ge powder, Qingwen Baidu decoction and Da Yuan decoction are the basic formulations for Qifen syndrome of COVID-19. The main medication characteristics are clearing heat, entilating lung, removing toxicity and removing turbidity. It shows that removing toxicity and eliminating evil are the prescription thought in treating epidemic disease of traditional Chinese medicine.
Confused?
Me too!
What seems to emerge is this:
- ‘Herbalists and Co’ did not wait long to jump on the corona bandwagon.
- They managed to confuse not just you and me, but even politicians, presidents, and their advisers.
- They produced a plethora of articles implying that an endless array of herbs might be effective.
- In doing so, no clear consensus emerged as to which herbs are the most promising.
- Sound evidence seems to be not available.
- Clinical trials are slow to start or not even planned.
- Everything is based on more or less wild extrapolation.
- Much of what is being published is borderline irresponsible.
- YET, IT MUST BE GOOD FOR BUSINESS!
Turmeric is certainly a plant with fascinating properties; we have therefore discussed it before. Reseach into turmeric continues to be active, and I will continue to report about new studies.
This study was aimed at estimating the effect of turmeric supplementation on quality of life (QoL) and haematological parameters in breast cancer patients who were on Paclitaxel chemotherapy. In this case series with 60 participants, QoL was assessed using a standard questionnaire and haematological parameters were recorded from the patients’ hospital records.
Turmeric supplementation for 21 days resulted in clinically relevant and statistically significant improvement in global health status, symptom scores (fatigue, nausea, vomiting, pain, appetite loss, insomnia), and haematological parameters.
The authors concluded that turmeric supplementation improved QoL, brought about symptom palliation and increased hematological parameters in breast cancer patients.
Really?
The way the conclusions are phrased, they clearly imply that turmeric caused the observed outcomes. How certain can we be that this is true?
On a scale of 0 -10, I would say 0.
Why?
Because there are important other determinants of the outcomes:
- placebo,
- concommittant treatments,
- natural history,
- etc., etc.
Why does this matter?
- Because such unwarranted conclusions mislead patients, healthcare professionals and carers.
- Because such bad science gives a bad name to clinical research.
- Because this type of nonsense might deter meaningful research into a promising subject.
- Because no ‘scientific’ journal should be permitted to publish such nonsense.
- Because it is unethical of ‘scientists’ to make false claims.
But maybe the Indian authors are just a few well-meaning and naive practitioners who merely were doing their unexperienced best? Sadly not! The authors of this paper give the following affiliations:
- Clinical Pharmacology, Pfizer Healthcare Private Limited, Chennai, Tamil Nadu, India.
- Department of Radiation Oncology, Faculty of Medicine, Sri Ramachandra Institute of Higher Education and Research, Porur, Chennai, Tamil Nadu, India.
- Process Development, HCL Technologies, Chennai, Tamil Nadu, India.
- Department of Pharmacognosy, Faculty of Pharmacy, Sri Ramachandra Institute of Higher Education and Research, Porur, Chennai, Tamil Nadu, India.
Yes, they really should know better!
So-called alternative medicine (SCAM) is, as we all know, an umbrella term. Under this umbrella, we find hundreds of different modalities that have little in common with each other. Here I often focus on:
- homeopathy,
- chiropractic,
- acupuncture,
- herbal medicine.
There are uncounted others, and in my recent book, I published critical evaluations 150 of them. But for the moment, let’s keep to the 4 SCAMs listed above.
What strikes me regularly is that many SCAM enthusiasts do seem to appreciate my critical assessments of SCAM; for instance:
- When I point out that the assumptions of homeopathy fly in the face of science, most SCAM enthusiasts agree.
- When I point out that chiropractic spinal manipulations might not be safe, most SCAM enthusiasts agree.
- When I point out that acupuncture is not a panacea, most SCAM enthusiasts agree.
- When I point out that herbal remedies can interact with prescribed drugs, most SCAM enthusiasts agree.
Most but not all!
- Those who find my criticism of homeopathy unfair are the homeopaths and their proponents.
- Those who find my criticism of chiropractic unfair are the chiropractors and their proponents.
- Those who find my criticism of acupuncture unfair are the acupuncturists and their proponents.
- Those who find my criticism of herbal medicine unfair are the herbalists and their proponents.
Hardly ever does a herbalist defend homeopathy’s weird assumptions; rarely does an acupuncturist tell me that I am too harsh with the chiropractors; never have I heard a chiropractor complain that my criticism of acupuncture is unjustified.
Entirely obvious?
Perhaps!
But I find it nevertheless curious, because my critical stance is always the same. I do not change it for this or that form of SCAM (I would also not change it for conventional medicine, but I leave it to those who have more specific expertise to do the criticising). I have no axe to grind against any particular SCAM. All I do is point out flaws in their logic, limitations in their studies, gaps in the evidence. All I do is provide my honest interpretation of the evidence.
It really seems to me that everyone appreciates my honesty, until I start being honest with them.
And this is why I find it curious. Homeopaths, chiropractors, acupuncturists, herbalists and all the other types of SCAM practitioners like to be seen on the side of science, evidence, critical thinking and progress. This, I suppose, is good for the (self) image; it might even help the delusion that they are all evidence-based. But as soon as someone applies science, evidence, critical thinking and progress to their very own little niche within SCAM, they stop liking it and start aggressing the critic.
I suppose this is entirely obvious as well?
Perhaps!
But it also exposes the double standard that is so deeply ingrained in SCAM.
We live in truly grim times! Let me therefore try to cheer you up a little. Here is a story that might make you smile.
In 1981, I moved back from London to Munich. While still in London, I had written an article on garlic for a German medical journal. It was published just as we arrived in our new home. Here is it’s English abstract:
Garlic has had a firm place in folk medicine since ancient times. More recent results are summarized here which show that extracts of the plant have an antimicrobial action, they are capable of lowering blood cholesterol and of reducing secondary vascular changes. They raise fibrinolytic activity and inhibit thrombocyte aggregation. Therefore the plant contains highly active therapeutic principles which appear to be particularly suitable for prophylaxis of arteriosclerosis.
Yes, you are quite right, this paper is nothing to write home about. So, why do I consider it ‘most consequential‘? Here is what happened:
My wife and I had barely arrived in our new home, when a man phoned (he had gone to a lot of trouble to find my number) and said: “I know you are the leading expert on garlic; I urgently need to talk to you”. Never correct a man’s mistake, if it’s in your favour, I thought, and we made an appointment for a meeting at the Munich train station hotel.
When I met him a few days later, he ordered me a coffee (which later I had to pay for) and explained that he had worked his whole life (he was about 50, I guessed) for the pharmaceutical industry and had now decided that this was enough. He thus planned to set up his own pharmaceutical company. He already had a photocopy machine in his basement, he proudly told me, and a wife who was willing to work as hard as he was. Specifically, his plan was to launch a garlic pill, and for that he needed my advice. I told him what he wanted to know, and we parted after about two hours promising to stay in contact.
The man’s name was Kuno Lichtwer.
During the weeks that followed, he often phoned me to pick my brain. One day, he told me that he had everything in place: he had found a supplier of the materials, a manufacturer to produce the pills and even registered a name for it:
KWAI

Then he popped the question that was foremost on his mind: ‘What do you think, Dr Ernst, should I risk it and go ahead with this or not?’. I had started to like that man; he was going to lose all his savings on a crazy idea, I felt. So, I told him: ‘If I were you, I would not do it. There are already plenty of garlic pills on the market. You are risking to lose everything.’ Then there was a long pause; eventually, he thanked me for my honest advice and hung up.
Weeks later he phoned again to tell me that he had truly appreciated my brutally direct advice, thought long and hard about it, but went ahead with his plan anyway. Would I now accept the position of ‘medical advisor’ to Lichwer Pharma? I was surprised, but accepted this new post. Thereafter, I advised him the best I could. We even conducted and published the very first clinical trial with his product. It was a rather flimsy study (we had no funds at all), but did suggest a positive result.
Each time Mr Lichtwer called me, he was elated; things were not just going well, they were booming! He was evidently hugely gifted in promoting KWAI. Then he invited me several times to come to Berlin where Lichtwer Pharma was based for business meetings. Proudly, he showed me that meanwhile his firm had moved out of his basement into a proper building. The next I knew was that he had a dozen employees. Lichtwer seemed unstoppable. This went on for 2 or 3 years, if I remember correctly.
During all this time, we had never talked about money, and my work for him had always been unpaid – that is, until one day just before Christmas he phoned and explained that he had moved his firm to yet a bigger building and hired yet more staff. He also realised that I deserved some renumeration for my advice; therefore, he had put a cheque in the post. When I told my wife about it, we both celebrated in anticipation of the substantial windfall. Two days later, his letter arrived. He very kindly thanked me for years of work and included a cheque of 500 DM (about 150 DM per year of work). A few months later, his firm had grown so big that a full time medical and research director was badly needed. He informed me that he had found a highly experienced expert and invited me to meet the new man, Prof Schulz.
No, I did not feel hard done by! On the contrary, I was happy that my prediction had been grossly wrong and that my friend Kuno was doing so well. In addition, I was also relieved, because my research at the University did not give me nearly enough time to look adequately after the now substantial firm of Lichtwer Pharma.
Thereafter, Lichtwer’s garlic pill went from strength to strength. Several larger studies confirmed our initial results that garlic positively influenced blood lipids (in 2000, our systematic review concluded: The available data suggest that garlic is superior to placebo in reducing total cholesterol levels. However, the size of the effect is modest, and the robustness of the effect is debatable. The use of garlic for hypercholesterolemia is therefore of questionable value). One day, I read somewhere that KWAI had become the most consumed pill in Germany (even beating Aspirin). Then Lichtwer Pharma went international and added several further herbal products to its portfolio. In 1991, Lichtwer Pharma was estimated to be worth 100 Million DM. Several years later, the firm had almost 400 employees and a yearly turnover of 353 Million DM.
To his credit, Kuno Lichtwer never entirely forgot me. When I had moved to the UK, he even came to Exeter, was entertained by my University, and made a donation of £100 000 towards a ‘Lichtwer Research Fellowship’ for my department. I am not sure whether Kuno Lichtwer is still alive. If he is, he would probably agree that, had I offered him 10 000 DM of my savings during our 1st meeting in 1981 (he did hint at that possibility), he would have gladly made me a partner in his enterprise.
But, as they say: money is not everything.
And a good story to tell is also not bad.
Hesperidin is a flavonoid found in citrus fruits, especially orange and grapefruit. It is said to have antioxidant and anti-inflammatory effects. Research into hesperidin began in the 1940s but only recently interest turned buoyant, and all sorts of benefits have been suggested. Here are just three recent clinical studies:
- This study investigated the effects of chronic intake of an orange extract (2S-hesperidin) or placebo on non-oxidative/glycolytic and oxidative metabolism markers and performance markers in amateur cyclists. A double-blind, randomized, placebo-controlled trial was carried out between late September and December 2018. Forty amateur cyclists were randomized into two groups: one taking 500 mg/day 2S-hesperidin and the other taking 500 mg/day placebo (microcellulose) for eight weeks. All participants completed the study. An incremental test was used to evaluate performance, and a step test was used to measure oxygen consumption, carbon dioxide, efficiency and oxidation of carbohydrates and fat by indirect calorimetry. The anaerobic power (non-oxidative) was determined using Wingate tests (30 s). After eight weeks supplementation, there was an increase in the incremental test in estimated functional threshold power (FTP) (3.2%; p ≤ 0.05) and maximum power (2.7%; p ≤ 0.05) with 2S-hesperdin compared to placebo. In the step test, there was a decrease in VO2 (L/min) (-8.3%; p ≤ 0.01) and VO2R (mL/kg/min) (-8.9%; p ≤ 0.01) at VT2 in placebo. However, there were no differences between groups. In the Wingate test, there was a significant increase (p ≤ 0.05) in peak and relative power in both groups, but without differences between groups. Supplementation with an orange extract (2S-hesperdin) 500 mg/day improves estimated FTP and maximum power performance in amateur cyclists.
- In this clinical trial with a parallel-group design, 49 patients with MetS received either 500-mg hesperidin or placebo, twice daily, for 12 weeks. Number of participants with treated MetS was considered as a primary end point. Anthropometric parameters, dietary intake, physical activity, lipid profile, glucose homeostasis parameter, tumor necrosis factor alpha (TNF-α), high-sensitivity C-reactive protein (hs-CRP) were assessed at the beginning and at the end of the study. Compared with the placebo group, hesperidin decreased fasting glucose level (- 6.07 vs. – 13.32 mg/dL, P = 0.043), triglyceride (- 8.83 vs. – 49.09 mg/dL, P = 0.049), systolic blood pressure (- 0.58 vs. – 2.68 mmHg, P = 0.048) and TNF-α (- 1.29 vs. – 4.44 pg/mL, P = 0.009). Based on the within-group analysis, hesperidin led to significant decrease in serum levels of glucose, insulin, triglyceride, total cholesterol, low density lipoprotein cholesterol, TNF-α and hs-CRP, while in control group only glucose and insulin significantly decreased. The results indicate that hesperidin supplementation can improve metabolic abnormalities and inflammatory status in patients with MetS.
- In this study, 64 patients were randomly allocated to receive 500 mg/day hesperidin or placebo capsules for 6 weeks. Data on systolic blood pressure (SBP), diastolic blood pressure, serum total antioxidant capacity (TAC), tumor necrosis factor alpha, interleukin 6 (IL-6), and high-sensitivity C-reactive protein (hs-CRP) were collected at the baseline and at the end of the study. In the hesperidin group, SBP (122.7 ± 8.5 vs. 119.0 ± 7.4; p = .005), mean arterial blood pressure (94.2 ± 5.5 vs. 91.8 ± 5.5; p = .009), IL-6 (8.3 ± 2.1 vs. 7.4 ± 1.8; p = .001), and hs-CRP (1.9 ± 1.2 vs. 1.1 ± 0.9; p < .000) decreased whereas TAC increased (0.74 ± 0.1 vs. 0.82 ± 0.1; p < .000) in comparison to the baseline values. There was a significant difference in mean percent change of SBP, diastolic blood pressure, mean arterial blood pressure, serum TAC, and inflammatory markers (tumor necrosis factor alpha, IL-6, and hs-CRP) between hesperidin and control groups following intervention in adjusted models (p < .05). These results suggest that hesperidin may have antihypertensive and anti-inflammatory effects in type 2 diabetes.

The latest suggestion for Hesperidin is – how could be be otherwise? – that it helps against COVID-19: Hesperidin can block coronavirus from entering host cells through ACE2 receptors which can prevent the infection. Anti-viral activity of hesperidin might constitute a treatment option for COVID-19 through improving host cellular immunity against infection and its good anti-inflammatory activity may help in controlling cytokine storm. Hesperidin mixture with diosmin co-administrated with heparin protect against venous thromboembolism which may prevent disease progression. Based on that, hesperidin might be used as a meaningful prophylactic agent and a promising adjuvant treatment option against SARS-CoV-2 infection.
According to one source, Hesperidin can cause several problems:
- abdominal pain,
- diarrhea,
- contact dermatitis,
- nausea,
- interactions with medications (including anticoagulants, blood pressure drugs, and calcium channel blockers),
- increased risk of bleeding.
No doubt, Hesperidin is an interesting substance. Yet, I feel that much more research is needed until we can be reasonably sure that it is clinically effective for any condition, particularly COVID-19.
It has been reported that Karnataka’s Deputy Chief Minister, Dr CN Ashwathnarayan, has launched eight products, several of which fall in the category of so-called alternative medicine (SCAM), aimed at mitigating COVID-19, developed by various start-ups at Bangalore Bioinnovation Centre (BBC). Dr CN Ashwathnarayan said the launch of the products shows that Karnataka has emerged as a leading state in developing solutions to fight the COVID 19 pandemic.
Here are short descriptions of the innovations:
- Padma Vitals +: Developed by Innovator start-up Dr. Madan Gopal of Cardiac Design labs,Padma Vitals + is a centralized monitoring system for ECG, respiration, Spo2 and body temperature, which can measure the vitals continuously and the analysis sent through telemetry, with an alerting system embedded in it. The device is much needed for contactless monitoring of patients during COVID 19 Pandemic. The product has been validated at Narayana Hrudayalaya.
- Malli’s Cordytea: Developed by Dr. Moushmi Mondal from Mallipatra Neutraceuticals, this product is an Immunity booster tea prepared from medicinal mushroom – Cordyceps. The mushroom variety grown under laboratory conditions is developed by the Innovator. Cordicepin, an active ingredient is known to have anti-viral properties too. In the COVID 19 times, it will be helpful in boosting the immunity levels. The product has been patented and is approved by FSSAI.
- CD4 Shield : Developed by Dr. Vijay Lanka and his team from Stabicon, this product is a chewable tablet containing curcumin and Vitamin B12. Both the ingredients fight inflammation and infection. The product ensures activation of innate immunity by activating CD4+, CD8+ and IFN 1 to virus specific effect and has immunomodulatory properties. It also reduces cytokine storm in response to viral infection. The product is approved by FSSAI.
- BeamRoti : Developed by Dr. Srinivas from Aspartika, the product is an immunity booster chapati having mixture of herbs recommended by AYUSH ministry. The ingredients have been prepared using supercritical fluid extraction technology to ensure optimum concentration of herbal extract reaches the body. The chapatis are easy to store with good shelf life and Patent application has been filed. The product is approved by FSSAI.
- Immune booster daily drops: Developed by Dr. Srinivas from Aspartika, the product is an immunity booster drop having mixture of herbs recommended by AYUSH ministry. The ingredients have been prepared using supercritical fluid extraction technology to ensure optimum concentration of herbal extract reaches the body by mixing just one drop of the product in a glass of hot water. The product is approved by FSSAI.
- VegPhal – Fruit and Vegetable Sanitizer: Developed by Deepak Bhajantri from Krimmi Biotech, this fruit and vegetable sanitizer is prepared using edible ingredients effective against microbes and removal of pesticides. It is chorine and alcohol free.
- Water Sanitizer – Kitchen Tap: The product is developed by Ravi Kumar from Biofi and is a miniaturized version of UV purifier that can be attached to a water tap and kill 99% of microbes including viruses such as phages.
- nti-Micobial HVAC module: The product is developed by Ravi Kumar from Biofi and is a module that can be fitted to HVAC system to ensure circulating air is sanitized. This is especially useful during COVID 19 times as many enclosed spaces in which AC circulated air may be contaminated. Based on UV-silver titanium dioxide technology, the product is patented and has been validated.
Karnataka is of course a state in the south western region of India. The region has so far about one million COVID-19 cases, while almost 12 000 people have died. One would therefore very much hope that the newly launched innovations can make a difference.
But will they?
As far as the SCAM-related products (e.g. ‘immune boosters’) are concerned, I see no convincing evidence to assume that they are effective. If anyone has information to the contrary, please let me know.
But why not? They can’t do any harm!
Sadly, I am am not so sure. I see the potential for considerable harm from all the useless SCAMs that are being promoted left right and centre for protecting the public against COVID-19. Firstly, there is the financial harm of paying for products that are useless. Secondly, ineffective effords might distract from finding and adhering to efforts that are effective. Thirdly, believing in a SCAM that does not work will create a sense of false security which, in turn, renders consumers more vulnerable to catch the virus.
As always in healthcare, even harmless interventions that do not work can become dangerous, as they lead to neglecting effective measures. I shudder to think of how many deaths have been caused by the many SCAM merchants who see the current pandemic as an opportunity.
In these pre-Xmas days, many homes will smell of cinnamon. It’s certainly a wonderful spice for creating an atmosphere. But ther are also other uses for ciannamon.
Current treatments for overactive bladder (OAB) have limited efficacy, low persistence and a high rate of adverse events commonly leading to treatment cessation in clinical practice. Clinicians in Asia commonly use traditional Chinese medicine as an alternative for OAB treatment despite it having uncertain efficacy and safety. To evaluate the efficacy and safety of cinnamon patch (CP) treatment for alleviating symptoms of OAB, this double-blind randomized, placebo-controlled trial was conducted.
The 6-week study was conducted in an outpatient setting; 66 subjects diagnosed as having OAB were enrolled and treated with a placebo (n=33) or CP (n=33). The OAB symptom score (OABSS) was selected as the primary end point, and a patient perception of bladder condition (PPBC), an urgency severity scale (USS), and post-voiding residual urine (PVR) volume were selected as secondary end points.
In total, 66 participants (40 women and 26 men), 60 years of age, were included in the intention-to-treat analyses. Baseline characteristics were comparable between the CP and placebo groups. Treatment with a CP showed statistically significant differences in reductions in OABSS scores, PPBC scores, and USS scores.
The authors concluded that compared to a placebo, treatment with CP might be considered an effective and safe complementary therapy for OAB. Further studies employing a positive control, different dosage forms, larger sample sizes, and longer treatment periods are warranted.
Cinnamon (Cinnamomum zeylanicum and Cinnamon cassia)belongs to the Lauraceae family. It contains manganese, iron, dietary fiber, and calcium as well as cinnamaldehyde, cinnamic acid, cinnamate, and numerous other components such as polyphenols and antioxidant, anti-inflammatory, antidiabetic, antimicrobial, anticancer effects. Several reports have dealt with the numerous properties of cinnamon in the forms of bark, essential oils, bark powder, and phenolic compounds, and each of these properties can play a key role in human health.
The new study is interesting and prompts me to ponder:
- Do the pharmacologically active ingredients of cinnamon pass the skin barrier in sufficient amounts to have any effect at all? Or perhaps it was the scent? In which case, this would have been a study of aromatherapy.
- Considering the typical scent of cinnamon, I find it hard to imagine that this study was truly double blind.
- Cinnamon is alleged to have antimicrobial, antiviral, antifungal, antioxidant, antitumor, antihypertensive, antilipemic, antidiabetic, gastroprotective, and immunomodulatory effects. I do wonder which, if any, of these are responsible for the observed clinical results of this trial.
- Cinnamon is known to sometimes lead to allergic reactions. I wonder whether this could be a problem when it is applied in patches.
So, for the time being, I think, I prefere cinnamon, the spice, to cinnamon, the medicine.
Alzheimer is a devastating condition. Despite much research, we are still far from being able to effectively prevent or treat it. Some claim that relatively simple dietary interventions might work. What does the evidence tell us?
The aim of this systematic review was to evaluate the effect of dietary interventions on the cognitive performance of individuals with Alzheimer’s disease (AD). Thirty-two RCT could be included.
The findings show that a wide range of supplements have been submitted to testing in RCTs. Most of the supplements seem to be less than useful. However, some seem to show some promise:
- Omega-3 fatty acid has positive effects at different doses.
- ‘Fortasyn Connect’ (a multi-nutrient mixture) seems to be effective in the early stages of the disease.
- Probiotic, Ginseng, Inositol and specialized nutritional formulas seem to have a positive effect on cognition.
Most of the primary studies had poor methodological quality, included patients with mild AD, small samples, and did not obtain significative results for all the cognitive outcomes.
The authors concluded that the effect of most dietary interventions on cognition in AD patients remains inconclusive, however, several nutrients, isolated or not, show potential to improve cognitive function in AD, especially in its early stages.
I am relieved that the authors of this thoroughly-researched review phrased their conclusions as cautiously as they did. The thing is, most of the primary trials are truly not worth writing home about. Some are just 4 weeks long, others include merely 30 odd patients. Many look more like marketing excercises than science.
The authors also stated that better quality studies are urgently needed to confirm the therapeutic potential of the diet so that a dietary recommendation in AD that contributes to the quality of life of patients and relatives can be established. This has become almost a standard sentence for ending a scientific paper. In this instance, however, it seems very true.