case report
It has been reported that a UK Conservative candidate for the next general election reportedly claimed she healed a man’s hearing through the power of prayer. Kristy Adams has been chosen to represent the Conservatives in Mid Sussex at the next general UK election, which is expected to take place in May or the autumn of next year. Mrs Adams previously stood as the Tory candidate in Hove in 2017, placing a distant second behind Labour MP Peter Kyle.
In a recording from 2010, the Conservative hopeful reportedly told the King’s Arms Church in Bedford how she healed a deaf man by placing her hands over his ears and saying: “Be healed in Jesus’s name”. Mrs Adams is reported to have said: “He had hearing aids in both ears and I just thought that wasn’t right. It just annoyed me. I said ‘can I pray for you?’ and his eyes lit up, which is unusual when you offer to pray for someone’s healing.” After removing her hands, she claims the man could hear without his hearing aids. “I don’t know if he was more surprised or me,” she reportedly said.
Speaking to The Argus during her 2017 election campaign, Mrs Adams said she had asked the Daily Mirror to remove a story about the alleged recording but refused to answer whether she believed non-scientific medical miracles can happen. She said: “Millions of Christians around the world pray every day to help people.”
- Daily prayer against severe COVID – an update of a study started two years ago
- Resolution of blindness after prayer?
- Prayer as a therapy: a new randomised study
- Prayer as a medical therapy? Time to stop this nonsense!
- When an undercover journalist tests alternative cancer healers
- Biblical Naturopathy, another SCAM that is new to me
- The ‘Association of Catholic Doctors’ and homeopathic conversion therapy
- Prof Harald Walach’s new ground breaking study of praying the Rosary
- Higher religiousness/spirituality is associated with a more frequent use of so-called alternative medicine (SCAM)
- ‘The power of all religions’ is being tested in a study with severely ill corona-virus patients
- Does religiosity influence post-operative survival?
- Daniel P Wirth, his dubious research, and the remarkable apathy of some medical journals
Suffice to say, perhaps, that the evidence for prayer as a therapy is not positive.
Homeopathic remedies are highly diluted formulations without proven clinical benefits, traditionally believed not to cause adverse events. Nonetheless, published literature reveals severe local and non–liver-related systemic side effects. Here is the first series on homeopathy-related severe drug-induced liver injury (DILI) from a single center.
A retrospective review of records from January 2019 to February 2022 identified 9 patients with liver injury attributed to homeopathic formulations. Competing causes were comprehensively excluded. Chemical analysis was performed on retrieved formulations using triple quadrupole gas chromatography-mass spectrometry and inductively coupled plasma atomic emission spectroscopy.
Males predominated with a median age of 54 years. The most typical clinical presentation was acute hepatitis, followed by acute or chronic liver failure. All patients developed jaundice, and ascites were notable in one-third of the patients. Five patients had underlying chronic liver disease. COVID-19 prevention was the most common indication for homeopathic use. Probable DILI was seen in 77.8%, and hepatocellular injury predominated (66.7%). Four (44.4%) patients died (3 with chronic liver disease) at a median follow-up of 194 days. Liver histopathology showed necrosis, portal and lobular neutrophilic inflammation, and eosinophilic infiltration with cholestasis. A total of 29 remedies were consumed between 9 patients, and 15 formulations were analyzed. Toxicology revealed industrial solvents, corticosteroids, antibiotics, sedatives, synthetic opioids, heavy metals, and toxic phyto-compounds, even in ‘supposed’ ultra-dilute formulations.
The authors concluded that homeopathic remedies potentially result in severe liver injury, leading to death in those with underlying liver disease. The use of mother tinctures, insufficient dilution, poor manufacturing practices, adulteration and contamination, and the presence of direct hepatotoxic herbals were the reasons for toxicity. Physicians, the public, and patients must realize that Homeopathic drugs are not ‘gentle placebos.’
The authors also cite our own work on this subject:
A detailed systematic review of homeopathic remedies-induced adverse events from published case reports and case series by Posadzski and colleagues showed that severe side effects, some leading to fatality, are possible with classic and unspecified homeopathic formulations. The total number of patients included was 1159, of which 1142 suffered adverse events directly related to homeopathy. The direct adverse events had acute pancreatitis, severe allergic reactions, arsenical keratosis, bullous pemphigoid, neurocognitive disorders, sudden cardiac arrest and coma, severe dyselectrolytemia, interstitial nephritis, kidney injury, thallium poisoning, syncopal attacks, and focal neurological deficits as well as movement disorders. Fatal events involved advanced renal failure requiring dialysis, toxic polyneuropathy, and quadriparesis. The duration of adverse events ranged from a few hours to 7 months, and 4 patients died. The authors state that in most cases, the mechanism of action for side effects of homeopathy involved allergic reactions or the presence of toxic substances—the use of strong mother tinctures, drug contaminants, adulterants, or poor manufacturing (incorrect dilutions).
When we published our paper back in 2012, it led to a seies of angry responses from defenders of homeopathy who claimed that one cannot ‘have the cake and eat it’; either homeopathic remedies are placebos and thus harmless, or they have effects and thus also side-effects, they claimed. As the new publication by Indian researchers yet again shows, they were mistaken. In fact, homeopathy is dangerous in more than one way:
- the homeopathic remedies can do harm if not diluted or wrongly manufactured;
- the homeopaths can do harm through their often wrong advice in health matters;
- homeopathy erodes rational thinking (as, for instance, the resopnses to our 2012 paper demonstrated).
The case of a 91-year old male patient developing acute neuropathic pain along the sciatic nerve distribution following spinal manipulation has been reported. Manipulative treatment with an Activator Adjusting Instrument (AAI) had been performed. During this treatment, three applications of the AAI were administered. The applications were bilateral (1) over the sacroiliac joint, (2) gluteal area, and (3) paraspinal region just above the iliac crest.
Within 24 hours, the patient developed severe 10/10 pain originating from the left gluteal area at the site of one of the activator deployments with radiation all the way down his left leg to the foot. He was able to maintain distal left leg strength and sensation. Subsequently, the patient developed insomnia, confusion, and adrenal gland dysfunction in response to changes in steroids, gabapentin, and other drugs, thus highlighting some nuances of managing elderly patients with back pain.
Relief was achieved with subsequent physical therapy techniques aimed at relaxing the patient’s deep gluteal muscles, raising the hypothesis of temporary injury to the deep gluteal muscles, with painful contractions resulting in gluteal region pain as well as sciatic nerve inflammation as the nerve passed through that region.
The authors concluded that this clinical case illustrates some of the perils and risks of spinal manipulation, particularly in the elderly, and the need for careful patient selection.
The authors of this (stranely incomplete) case report discuss whether any manipulation was truly necessary or indicated as part of his initial chiropractic treatment plan. They state that, given that complications associated with similar practices are not often reported in the literature, this case highlights important considerations to be made in the elderly given the potential impact of transient/permanent neuropathic pain in that population subset.
Somehow, I doubt that we can be certain that the patient improved due to the physical therapy and not due to the drugs he received. Moreover, I question the authors’ repeated assertions that such adverse effects of chiropractic spinal manipulation are truly rare. Here is a section from our own 2002 systematic review of the subject:
A systematic review of five prospective investigations of the risks of spinal manipulation concluded that mild-to moderate transient adverse reactions occur in approximately half of patients who undergo spinal manipulation. The largest of these studies involved 1058 patients who received a total of 4712 treatments from 102 chiropractors in Norway. At least one adverse reaction was reported by 55% (n 580) of patients. About one quarter (n 1174) of treatments resulted in at least one adverse reaction. The most common reaction reported was local discomfort. Eighty-five percent (n 824) of reactions were described as “mild or moderate” and 1% (n 14) as “unbearable.” Seventy-four percent (n 1052) of reactions disappeared within 24 hours. No serious, permanent complications of spinal manipulation were reported, but follow-up was not described. These results were confirmed by a similar study in Sweden with 625 patients and a smaller one (68 patients) from the United Kingdom …
Non-life-threatening adverse effects after spinal manipulations are not rare – they are merely rarely reported!
This short news report appeared on X [formerly Twitter]:
The Ohio State Medical Board just approved the indefinite suspension Dr. Sherri Tenpenny’s medical license, an osteopathic physician and longtime figure in the anti-vaccine movement. The board got around 350 complaints into her behavior, but that’s not why she’s being suspended.
As this could easily be unreliable, I looked for confirmations … and found several, for instance, this one:
An Ohio physician who sparked widespread ridicule in 2021 after spreading bizarre COVID-19 vaccine conspiracies to the House Health Committee by claiming the jabs magnetize their hosts and “interface” with cell towers had her medical license indefinitely suspended Wednesday. Anti-vaccine spreader Sherri Tenpenny sparked a firestorm in June, 2021 after making the comments, which saw 350 complaints sent to the State Medical Board. According to Cleveland.com, the board’s decision was not based on the comments, rather on procedural grounds, citing Tenpenny’s refusal to cooperate with investigators during the inquiry. “Dr. Tenpenny, neither you nor any doctor licensed by this board is above the law, and you must comply with the investigation,” said Dr. Jonathan Feibel, an orthopedic surgeon and medical board member, according to the outlet. “You have not done so, and therefore, until you do, your license will be suspended.” A lawyer for Tenpenny, Tom Renz, described the investigation as a form of “harassment” on her “free speech rights.” Tenpenny did not speak after the announcement, however Renz declared, “This appears very much like a lynch mob.”
Who is Sherry Tenpenny? Here is what Wiki tells us about her:
Sherri J. Tenpenny is an American anti-vaccination activist and conspiracy theorist who promulgates the disproven hypothesis that vaccines cause autism.[1] An osteopathic physician, she is the author of four books opposing vaccination. A 2015 lecture tour of Australia was canceled due to a public outcry over her views on vaccination, which oppose established scientific consensus. A 2021 Center for Countering Digital Hate analysis concluded that Tenpenny is among the top twelve people spreading COVID-19 misinformation and pseudoscientific anti-vaccine misinformation on social media platforms. She has falsely asserted that the vaccines magnetize people and connect them with cellphone towers…
The story is puzzling, in my view. The biggest question for me is this:
Why only now?
She should have been suspended years ago!
We have discussed the Miracle Mineral Solution (MMS) before. Now it has been making headlines again. It has been reported that a Miami federal jury convicted a father and his three sons of selling a toxic bleach solution as a “miracle” medical cure out of a fake Florida church’s website to thousands of consumers across the US. Mark Grenon, 65, and sons Jonathan, 37, Joseph, 35, and Jordan, 29, chose to represent themselves in their two-day trial in Miami federal court. But they said nothing during the trial as if they were silently protesting the proceeding. Only after the 12-person jury hit them with a quick verdict did one of the Grenons speak up. “We will be appealing,” Joseph Grenon said.
During the trial and closing arguments, prosecutors portrayed the four defendants as con men who used a phony religious front on a website, the Genesis II Church of Health and Healing, to sell $1 million worth of their “Miracle Mineral Solution” a cure for 95% of the world’s known diseases, from AIDS to the coronavirus. “This whole Miracle Mineral Solution scheme was built on deception and dishonesty,” the prosecutor said during his closing argument, telling jurors that the Grenons “created a fake church to make it harder for the Food and Drug Administration and government to stop them from selling snake oil.” But, “this was no church,” he argued. “This was a scam for money — an old-fashioned scam.” The jury found the four defendants — all wearing beige inmate uniforms, ponytails, and flowing beards — guilty of conspiring to defraud the U.S. government and FDA, which regulates the food and drug industry, by distributing an unapproved and misbranded drug, Miracle Mineral Solution (MMS). That conviction carries up to five years in prison.
During the trial, the prosecutor said the Grenons called themselves “bishops” and peddled MMS as “sacraments” to consumers in South Florida and other parts of the US in exchange for a “donation” to the Genesis church, before the FDA cracked down on the family in 2020.
The Grenons were charged that April with conspiring to defraud the U.S. government after the outbreak of the COVID-19 pandemic when they defied FDA and court orders to stop distributing the toxic MMS substance. Their criminal case was the first pandemic-related enforcement action in Florida. In public warnings, FDA said it received several reports of hospitalizations and life-threatening conditions as people drank the dangerous substance.
MMS is a chemical solution containing sodium chlorite that, when mixed with water and a citric acid “activator,” turns into chlorine dioxide, a powerful bleach typically used for industrial water treatment or bleaching textiles, pulp, and paper. During the trial, a FDA agent testified about three Grenon-produced videos that pitched the solution as a cure for cancer, lung cancer, and COVID-19, among other deadly diseases. “We are trying to create a world without disease,” Mark Grenon said in one video, pitching the MMS substance. “It’s been proven to be tremendously effective in curing cancer.” Another video, dated March 8, 2020, was titled: “The coronavirus is curable. Do you believe it? You better!”
Prosecutors said the Grenon family’s religious front, the Genesis II Church of Health and Healing, sold tens of thousands of MMS orders in violation of federal law since 2010. It was in that year that Mark Grenon claims to have founded the organization with a man named Jim Humble in a plan to avoid governmental regulation and arrest as they promoted MMS as a miracle cure. Humble, a man who has dabbled in Scientology and professed to be a billion-year-old god, began promoting the substance as early as 2006 in self-published works after he claimed to have discovered its medical properties while on a gold-mining expedition in South America. After Humble supposedly stepped away from the organization in 2017, Grenon continued to manufacture, promote and sell MMS with his three sons.
The Grenons’ open defiance of a court order ultimately led to criminal charges and a federal raid on the family’s Bradenton home, where federal investigators say they found loaded guns, nearly 10,000 pounds of sodium chlorite powder, and thousands of bottles of MMS.
We have discussed the currently fashionable herbal remedy, ‘kratom‘, before:
Inadequate regulation of Kratom supplements put consumers at risk
News about Kratom: the herb was recently (semi-)legalized in Thailand
Kratom: a ‘herbal drug’ with the potental to do more harm than good
A quick recap:
Kratom is made of the leaves of Mitragyna speciosa, a tree endogenous to parts of Southeast Asia. It has been used traditionally for its stimulant, mood-elevating, and analgesic effects. The plant’s active constituents, mitragynine and 7-hydroxymitragynine, have been shown to modulate opioid receptors, acting as partial agonists at mu-opioid receptors and competitive antagonists at kappa- and delta-opioid receptors. Both alkaloids are G protein-biased agonists of the mu-opioid receptor and therefore, may induce less respiratory depression than classical opioid agonists. The Mitragyna alkaloids also appear to exert diverse activities at other brain receptors (including adrenergic, serotonergic, and dopaminergic receptors), which may explain the complex pharmacological profile of raw kratom extracts. By the early 2000s, kratom was increasingly used in the US as a substitute for prescription and illicit opioids for managing pain and opioid withdrawal by people seeking abstinence from opioids. There are numerous assessments where people have been unable to stop using kratom and withdrawal signs and symptoms are problematic. Kratom does not appear in normal drug screens and, when taken with other substances of abuse, may not be recognized.
Now it has been reported that the family of a Florida woman who died in 2021 after ingesting kratom has been awarded more than $11m from a distributor of the herbal extract. “There is of course no amount of money that will make up for the pain and suffering that Ms Talavera’s children are enduring because of their mother’s death,” Middlebrooks wrote in court records addressing the sanction against Kratom Distro. “The law nonetheless recognizes that the defendant must pay something, however inadequate.”
The US Drug Enforcement Agency in 2016 had imposed its strictest restrictions on kratom, which is made from the leaves of an evergreen tree and is often used by people to self-treat pain, anxiety, depression, and opioid addiction as well as withdrawal. There was an intense, immediate public backlash to that approach, however, and it prompted the DEA to rescind its prohibition of kratom, which is sold in stores and online.
The US Food and Drug Administration nonetheless has warned consumers over possible safety and addiction risks associated with kratom, and it has spoken in favor of more research aimed at gaining a better understanding of “the substance and its components”.
Friends of Talavera, a resident of the Florida community of Boynton Beach, introduced her to kratom years before her death. Her family said she regarded it as a safe, natural supplement and had taken some after buying it online from the Kratom Distro when her partner and the father of her youngest child – Biagio Vultaggio – found her unconscious in the living room on 20 June 2021. The 39-year-old Talavera was face down on the ground next to an open bag of a kratom derivative marketed as a “space dust”, her family has said. Vultaggio called paramedics, and they took Talavera to a hospital where she was pronounced dead. An autopsy later listed Talavera’s cause of death as acute intoxication from mitragynine, the main kratom component. The local coroner wrote in a report that “at high concentrations, mitragynine produces opioid-like effects, such as respiratory failure”.
________________________
Kratom Disro claims that 
Product Consistency
- Our kratom powder is sourced directly from Indonesia monthly. Your order was literally on a farm in Indonesia two months ago. No old powder.
- Our kratom extracts are produced in the US by a licensed chemist and a professionally trained staff.
- We only use delicious flavors and quality ingredients.
Complete Transparency
- Every batch of products we receive is lab tested and will not ship out without meeting our meticulous quality standards.
- Current labs – We will never show you an out-of-date lab with our products.
- Guaranteed purity levels and free of all toxins.
Get It When You Want It
- Many orders shipped same day.
- USPS shipping on all orders under 6 pounds.
- Larger orders can ship USPS Priority for a small additional charge.
_________________________________
Back to the above lawsuit:
One of the attorneys for Talavera’s family, Tamara Williams, said in a statement that the judgment won by her clients “should be a wakeup call to the kratom industry”. Williams’s law firm had also recently won a $2.5m jury verdict against a kratom manufacturer in Washington state after a separate lawsuit alleging wrongful death. A colleague of Williams called on government officials to take steps “to protect other families from having to deal with unnecessary kratom overdose deaths”.
Sobrenix (Kudzu, Milk Thistle, B Vitamins & More) is “designed to reduce alcohol cravings and help you detoxify your body so you can successfully manage alcohol consumption. Even better, taken before drinking, Sobrenix’s ingredients help you stop before you’ve had too much. DETOXIFY YOUR BODY with a powerful formula that combines herbs and nutrients that support liver health, curb cravings, and help you wake up without a nasty hangover. Sobrenix kick-starts the detoxification process with essential herbs like Milk Thistle and Chanca Piedra. Additionally, the formula contains the critical B-Vitamins that alcohol washes away so you can wake up happy and healthy again!”
Yes, you suspected correctly: this is pure BS!
Not only that but the Federal Trade Commission is taking action under the FTC Act and the Opioid Addiction Recovery Fraud Prevention Act of 2018 (OARFPA) against the makers of Sobrenix. According to the FTC’s complaint, the makers, a company, Rejuvica, and its owners, Kyle Armstrong and Kyle Dilger, made numerous unsubstantiated and false claims about Sobrenix and used paid endorsers in deceptively formatted advertising. The defendants also used bogus review sites to deceive consumers about their products.
As a result of the FTC’s suit, the defendants have agreed to a proposed court order that would permanently ban them from making any unsubstantiated claims about healthcare products or services, as well as require them to pay $650,000 to the FTC to be used for providing refunds to consumers.
“We will not tire in our pursuit of those who prey on individuals struggling with alcohol or other substance use disorders,” said Samuel Levine, Director of the Bureau of Consumer Protection. “This case evidences the breadth of the FTC’s authority to pursue such wrongdoing under both the FTC Act and OARFPA.”
The FTC charges that the defendants marketed Sobrenix with messages like:
- “STRUGGLING TO CONTROL YOUR ALCOHOL CONSUMPTION? Sobrenix is designed to reduce alcohol cravings and help you detoxify your body so you can successfully manage alcohol consumption. Even better, taken before drinking, Sobrenix’s ingredients help you stop before you’ve had too much.”
The FTC charges that Rejuvica and its owners lacked adequate evidence to support these claims. The complaint charges that Rejuvica, Armstrong, and Dilger violated both the FTC Act and OARFPA. The proposed order contains a total monetary judgment of $3,247,737, which is partially suspended based on the defendants’ inability to pay the full amount. The defendants will be required to pay $650,000 to the FTC to be used to refund consumers. If the defendants are found to have lied to the FTC about their financial status, the full judgment will be immediately due.
______________________________
A few short comments might be in order:
- Regulators have the duty to protect consumers from false health claims.
- It is commendable that some authorities sometimes do their duty and go after some of the people responsible for making false claims related to dietary supplements.
- Such actions should, however, occur MUCH more often.
- They ought to happen also in countries other than the US.
- Similar actions should be initiated against ALL false claims made for healthcare products and services.
- This means that all practitioners of so-called alternative medicine (SCAM) would need to review their advertising, websites, etc., and erase therapeutic claims that are not supported by evidence.
- This would unquestionably amount to an enormously valuable service to public health.
- Most countries already have legislation that would make such steps possible; my question, therefore, is this:
WHY ARE CONSUMERS NOT ADEQUATELY PROTECTED BY THEIR NATIONAL REGULATORS FROM CHARLATANS WHO SELL INEFFECTIVE AND OFTEN DANGEROUS SCAMs AT HIGH COSTS?
As the organizer of several demos in the area of Linz, Austria, a ‘corona activist’ and ‘Holocaust denier’ had repeatedly made headlines over the past two years. Now the 39-year-old Austrian man is in the headlines yet again.
It has been reported that, on the evening of July 23, he was stopped by the police for a routine traffic control. His three children, aged 15, 11, and 5, were also in the car. “I know I’m wanted. I don’t have a driver’s license and I have a dead body in the trunk,” he said as he got out of the car. As the officers soon realized, he was only partly joking. A legal case for Holocaust denial was pending against the man who had not appeared at his main hearing last August, so a search was underway for him.
When police officers checked the car, they made the horrifying discovery. In the trunk was a woman’s body, wrapped in sheets. The dead woman turned out to be the wife, aged 38, of the driver. According to preliminary findings, she had died 4 hours earlier. Apparently, she had suffered from incurable cancer, and the police suspect that the illness had not been treated – her husband did not just not believe in vaccinations but disliked all drugs.
The husband, who already had several previous convictions, claimed that he was on his way to bury his wife somewhere “in nature”. The 39-year-old man was arrested and is now in pre-trial detention – though not for the incident with his wife’s body, but for Holocaust denial. He is said to have compared the Corona measures to the Holocaust, and the arrest order was issued because he failed to appear for his trial.
_________________________
One does not need to be a clairvoyant to predict that this remarkable man will come up with more surprises. I wonder what he might think of next.
In a video, Mr.Darkmoore speaks from a hospital bed and says the cause behind his visit to the emergency room stemmed from a chiropractor’s work on him. Three days ago, he had a ringing in his ear due to a long-term condition he knew as tinnitus. Thus, he decided to visit a chiropractor. “I figured $100 to a chiro, let them adjust a few things, if all else fails, I’ll go to a doctor,” explains Darkmoore.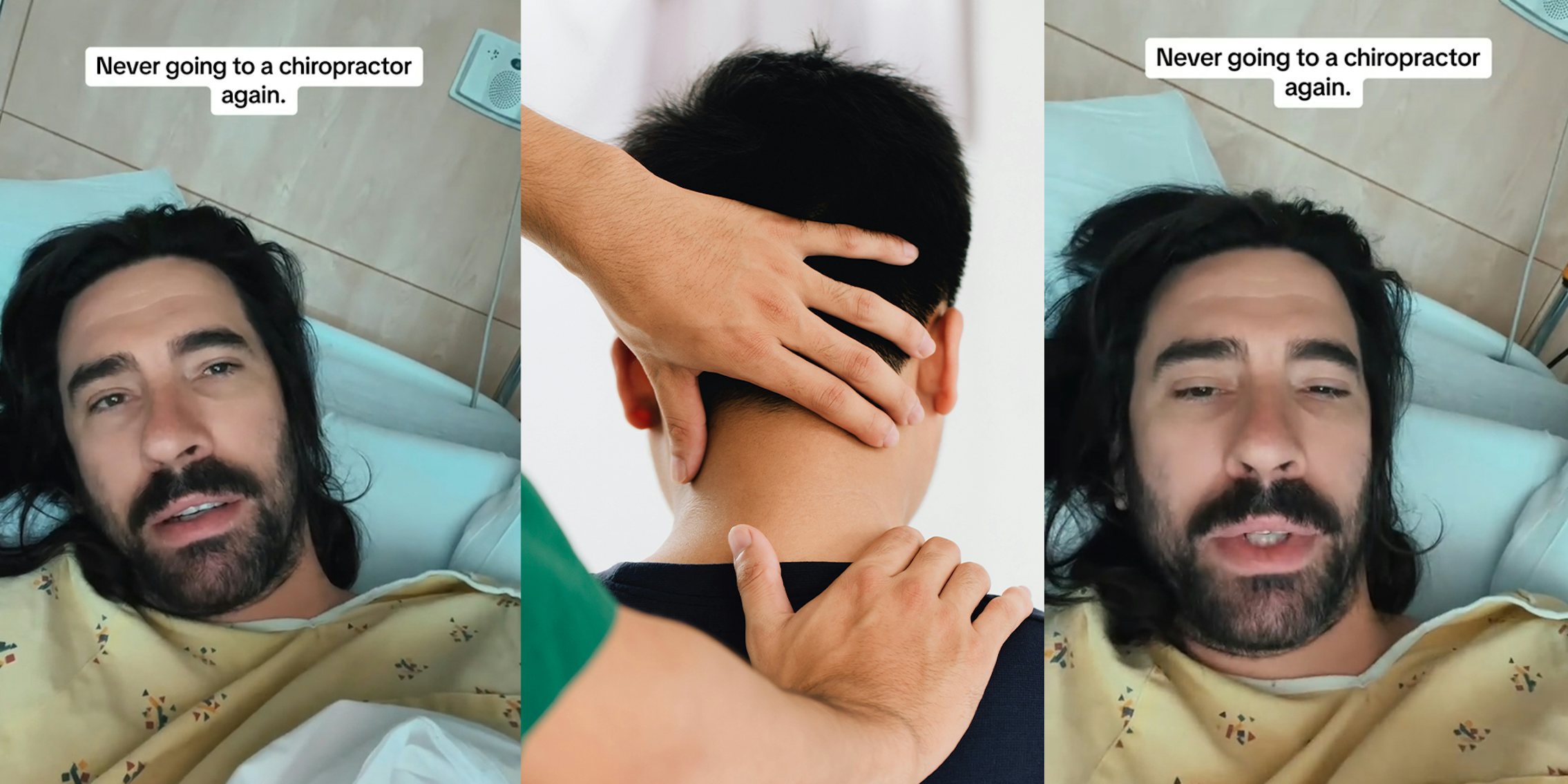
This $100 visit led to several other complications and doctor’s bills worth thousands of dollars. The day after he was treated by the chiropractor, he experienced a headache that eventually led to dizzy spells. He decided to visit the emergency room where a CT scan was ordered which showed that the chiropractor had dissected an artery in his neck.
Next, Darkmoore was put on blood thinners to avoid blood clots that could result in a stroke or worse. Darkmoore explains that he will be on two blood thinners for the next three months to prevent clotting. If the dissection heals partially, he says he will need to take aspirin every day for the rest of his life. If it doesn’t heal, he claims that he will need surgery.
Many viewers of the video claimed they have had the same “chiropractor gone wrong” experience as Darkmoore.
- “Wow. How scary. I had a similar thing happen to me. Extremely bad headache after going one time. Haven’t gone since,” one commenter wrote.
- “I’m so sorry this happened to u,” another user echoed. “My chiropractor also caused an injury which required emergency surgery & I have permanent damage. I’m glad u survived.”
Darkmore captioned his last update video, “I appreciate the thoughts and prayers. Hopefully, I’ll be okay after 3 months of recovery.”
__________________
Let’s hope that he is correct and that he will make a speedy and full recovery.
Of course, chiros will queue up to stress that important details are missing in this case report. To them, I would merely say this:
THERE IS NO GOOD EVIDENCE THAT NECK MANIPULATIONS BRING ANY BENEFIT AND QUITE A BIT OF EVIDENCE THAT THEY CAN CAUSE SERIOUS HARM.
SO, WHY NOT JUST STOP OFFERING THE PROCEDURE?
The U.S. Department of Health and Human Services alleges that Jason James of the James Healthcare & Associates clinic in Iowa, USA — along with his wife, Deanna James, the clinic’s co-owner and office manager — filed dozens of claims with Medicare for a disposable acupuncture device, which is not covered by Medicare, as if it were a surgically implanted device for which Medicare can be billed. According to the lawsuit, more than 180 such claims were filed. Beginning in 2016, the lawsuit alleges, the clinic began offering an electro-acupuncture device referred to as a “P-Stim.” When used as designed, the P-Stim device is affixed behind a patient’s ear using an adhesive. The device delivers intermittent electrical pulses through a single-use, battery-powered attachment for several days until the battery runs out and the device is thrown away.
Because Medicare does not reimburse medical providers for the use of such devices, DHHS alleges that some doctors and clinics have billed Medicare for the P-Stim device using a code number that only applies to a surgically implanted neurostimulator. The use of an actual neurostimulator is reimbursed by Medicare at approximately $6,000 per claim, while P-Stim devices were purchased by the Keokuk clinic for just $667, DHHS alleges. The department alleges James knew his billings were fraudulent as the P-Stim device is “nowhere close to even resembling genuine implantable neurostimulators” and does not require surgery.
The lawsuit alleges that on June 15, 2016, when Jason James was contemplating the use of P-Stim devices at the Keokuk clinic, he sent a text message to P-Stim sales representative Mark Kaiser, asking, “Is there a limit on how many Neurostims can be done on one day? Don’t wanna do so many that gives Medicare a red flag on first day. Thanks.” After realizing the “large profit windfall” that could result from the billing practice, DHHS’s lawsuit alleges, James “told Mark Kaiser not to mention the Medicare reimbursement rate to his nurse practitioner or staff – only his office manager and biller needed that information.” James then pressured clinic employees to heavily market the P-Stim devices to patients, even if those patients were not agreeable or, after trying it, were reluctant to continue the treatment, the lawsuit claims.
In October 2016, the clinic’s supplier of P-Stim devices sent the clinic an email stating the company had “no position on what the proper coding might be for this device if billed to a third-party payer” such as an insurer or Medicare, according to the lawsuit. The company advised the clinic to “consult a certified biller/coder and/or attorney to ensure compliance.” According to the lawsuit, James then sent Kaiser a text message asking, “Should we be concerned?”
DHHS alleges the clinic’s initial reimbursement claims were submitted to Medicare through a nurse practitioner and were denied for payment due to the lack of a trained physician’s involvement. In response, the clinic hired Dr. Robert Schneider, an Iowa-licensed physician, to work at the clinic for the sole purpose of enabling James Healthcare & Associates to bill Medicare for the P-Stim devices, the lawsuit claims. James then informed Kaiser he had a goal of billing Medicare for 20 devices per month, which would generate roughly $125,573 of monthly income, the lawsuit alleges. The lawsuit also alleges Dr. Schneider rarely saw clinic patients in person, consulting with them instead through Facebook Live.
In April 2017, Medicare allegedly initiated a review of the clinic’s medical records, triggering additional communications between James and Kaiser. At one point, James allegedly wrote to Kaiser and said he had figured out why Medicare was auditing the clinic. “Anything over $7,500 is automatically audited for my area,” he wrote, according to the lawsuit. “We are now charging $7,450 to remove the audit.”
The clinic ultimately submitted 188 false claims to Medicare seeking reimbursement for the P-Stim devices, DHHS alleges, with Medicare paying out $4,100 and $6,300 per claim, for a total loss of $1,028,800. DHHS is suing the clinic under the federal False Claims Act and is seeking trebled damages of more than $3 million, plus a civil penalty of up to $4.2 million.
An attorney for the clinic, Michael Khouri, said Wednesday he believe the federal government’s lawsuit was filed in error because a settlement in the case had already been reached. However, the assistant U.S. attorney handling the case said no settlement in the case had been finalized and the lawsuit was not filed in error.
Previous legal cases
In 2015, the Iowa Board of Chiropractic charged Jason James with knowingly making fraudulent or untrue representations in connection with his practice, engaging in conduct that was harmful or detrimental to the public, and making untruthful statements in advertising. The board alleged James told patients they would be able to stop taking diabetes medication through the use of a diet and nutrition program, and that he had claimed to be providing extensive laboratory tests when not all of the tests for which he billed were ever conducted. The board also claimed James referred patients to a medical professional who was not licensed to practice in Iowa. The case was resolved with a settlement agreement in which James agreed to pay a $500 penalty and complete 10 hours of education in marketing and ethics.
In 2019, Schneider sued the clinic for failing to comply with the terms of his employment agreement. Court exhibits indicate the agreement stipulated that Schneider was to work no more than two days per month and would collect $2,000 for each day worked, plus $250 per month for consulting, plus “$250 per device over six per calendar month.” In March 2020, a jury ruled in favor of the clinic and found that it had not breached its employment agreement with Schneider.
_________________________
Before some chiropractors now claim that such cases represent just a few rotten apples in a big basket of essentially honest chiropractors, let me remind them of a few previous posts:
- A $2.6M Insurance Fraud by Chiropractors and Doctors?
- Fraud and sex offences by chiropractors
- Chiropractic therapy for gastrointestinal diseases. Evidence of scientific misconduct?
- Twenty Things Most Chiropractors Won’t Tell You
- The Dark Side of Chiropractic Care
- Chiropractic subluxation: the myth must be kept alive
- CHIROPRACTIC: an early and delightful critique
- Students of chiropractic condemn the ‘unacceptable behaviour’ of some chiropractors and their professional organisations
- Far too many chiropractors believe that vaccinations do not have a positive effect on public health
- Chiropractor is in the dock for not wearing a mask
- “The uncensored truth” about COVID-19 vaccines” … as told by some chiro loons
To put it bluntly: chiropractic was founded by a crook on a bunch of lies and unethical behavior, so it is hardly surprising that today the profession has a problem with ethics and honesty.
