supplements
It has recently been reported that a Canadian naturopath claims he can treat autism with fecal transplants at a clinic in Mexico. The College of Naturopathic Physicians of B.C. has thus barred him stating that it has taken “extraordinary action” against Jason Klop in response to a complaint from a whistle-blowing former employee, who alleges that he manufactured these products in a “household lab” in B.C. without standard procedures or quality control.
While the complaint is under investigation, Klop cannot manufacture, advertise or sell fecal microbiota transplants (FMT). He’ll also be subject to random on-site audits to make sure he’s not violating his conditions.
This is the first public sign of concrete action by the college since CBC News reported on Klop’s business in January 2020 — nearly 20 months ago. Klop has been charging about $15,000 US for autistic children as young as two years old to have FMT treatment at a clinic near Tijuana. The process isn’t approved as a treatment of autism and carries serious risks of infection.
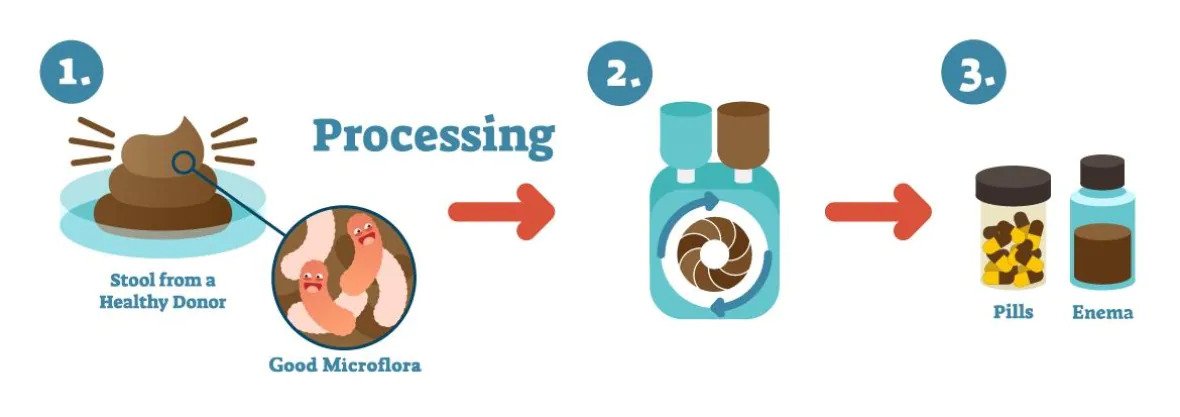
An illustration shows how fecal microbiota transplants are produced. (Vancouver Island Health Authority)
In a promotional video posted in January, Klop says he believes that “precision manipulation of the gut microbiome will solve every single chronic disease.” He also issued an affidavit boasting that he has a new lab that “produces the best and safest FMT materials in the world” and described the former employee who complained as “manifestly unreliable.” Klop argued that “lives are at stake” if he were to stop what he’s doing and described his therapy as a “life-saving measure.”
_____________________
Is there any evidence at all for FMT as a treatment of autism? A recent systematic review drew this conclusion: evidence from human studies suggesting beneficial effects of probiotic, prebiotic, and combination thereof, as well as fecal transplants in autism spectrum disorder, is limited and inconclusive.
Research on glucosamine, one of the most popular dietary supplements, shows anti-inflammatory and anti-cancer benefits with minimal adverse effects. An international team of researchers aimed to explore the relationship between the use of glucosamine and the risk of lung cancer and lung cancer mortality based on data from the large-scale nationwide prospective UK Biobank cohort study.
Participants were enrolled between the years 2006 and 2010 and followed up to 2020. The Cox proportion hazards model was used to assess the relationship between glucosamine use and the risk of lung cancer and lung cancer mortality. Subgroup analyses and sensitivity analyses were performed to explore the potential effect modifications and the robustness of the main findings.
A total of 439,393 participants (mean age: 56 years; 53% females) with a mean follow-up of 11 years were included for analyses. There were 82,603 (18.80%) participants reporting regular use of glucosamine at baseline. During follow-up, there were 1,971 (0.45%) lung cancer events documented. Glucosamine use was significantly associated with a decreased risk of lung cancer (hazard ratio=0.84, 95% CI: 0.75-0.92, p<0.001) and lung cancer mortality (hazard ratio=0.88, 95% CI: 0.81-0.96, p=0.002) in fully adjusted models. A stronger association between glucosamine use and decreased lung cancer risk was observed in participants with a family history of lung cancer when compared to those without a family history.
The authors concluded that regular use of glucosamine was significantly related with decreased risk of lung cancer and lung cancer mortality, based on data from this nationwide prospective cohort study.
A previous analysis of the same data concluded that regular glucosamine supplementation was associated with lower mortality due to all causes, cancer, CVD, respiratory and digestive diseases. The new analysis shows a strong association with lung cancer.
This is certainly interesting, but does it prove a causal relationship?
The answer is no.
Correlation is not causation!
What would be helpful in testing whether we are dealing with a cause-effect relationship?
- We need data from other studies. Several other epidemiological investigations indicated that glucosamine use might play a role in the prevention of cancer.
- We require to know the strength of the association. The new analysis suggests that it is indeed strong.
- We need a mode of action. Might the anti-inflammatory action of glucosamine explain the effect?
- We should ask whether there is a dose-response relationship. As far as I know, this has not been addressed as yet.
- Ideally, we would require a randomized trial to test the hypothesis. But I fear that such a study might be too difficult to conduct and will thus not be forthcoming.
And what if glucosamine should one day be proven to reduce the cancer risk? Would it become the first ALTERNATIVE measure to prevent cancer?
Certainly not!
It would automatically become a conventional method of cancer prevention. All the research into the subject has been entirely conventional and is unrelated to the alternative medicine movement. Or, to put it bluntly, alternative cancer prevention is a contradiction in terms. Either it works in which case it is conventional medicine, or it doesn’t in which case it is not even an alternative but at best so-called alternative medicine.
Bromelain, papain and chymotrypsin are proteolytic enzymes. They can be found in fruits such as pineapple or papaya, but also in the human body, namely in the pancreas. Besides their enzymatic functions, they have long been said to have a wide range of positive health effects. For instance, it is claimed that they reduce side effects and even improve the outcome of cancer therapies. This systematic review examined the existing evidence on the role that these enzymes which are available as food supplements might play in cancer treatment.
A total of 15 studies with 3,008 patients could be included in this systematic review. Patients treated with enzymes were diagnosed with various entities of gastrointestinal, gynecologic, head and neck, and lung cancer as well as hematological malignancies. The therapy concepts included mainly oral intake of enzymes in addition to conventional therapies. Investigated outcomes were:
- side-effects of anticancer therapy,
- quality of life,
- anticancer effects,
- survival rates.
Due to conflicting results and moderate quality of the included studies, the evidence is insufficient to attribute positive effects to enzymes in terms of better tolerability of the various antineoplastic therapies or even improvement in treatment efficacy. In most cases, enzyme therapy was well tolerated; side-effects were mainly gastrointestinal complaints such as diarrhea or meteorism.
The authors concluded that there is no clear therapeutic benefit of enzymes neither as supportive therapy nor as part of antineoplastic therapy.
I fully agree with this conclusion. In fact, in my new book that is just being published, I summarised the evidence for enzyme therapy (and many more alternative cancer therapies) in very similar terms: the evidence to suggest that enzyme therapy might be an effective treatment for any type of cancer is less than convincing.
I find it highly irresponsible to claim otherwise. Cancer patients are vulnerable and can easily be tempted to opt for one of the many quack treatments that are said to be both effective and free of nasty adverse effects. If they do try such options, they usually pay dearly, and not just in monetary terms.
This retrospective electronic medical record data analysis compared the characteristics and outcomes of drug-induced liver injury (DILI) caused by paracetamol and non-paracetamol medications, particularly herbal and dietary supplements. Adults admitted with DILI to the Gastroenterology and Liver Centre at the Royal Prince Alfred Hospital, Sydney (a quaternary referral liver transplantation centre), 2009-2020 were included. The 90-day transplant-free survival and the drugs implicated as causal agents in DILI were extracted from the records.
A total of 115 patients with paracetamol-related DILI and 69 with non-paracetamol DILI were admitted to our centre. The most frequently implicated non-paracetamol medications were:
- antibiotics (19, 28%),
- herbal and dietary supplements (15, 22%),
- anti-tuberculosis medications (6, 9%),
- anti-cancer medications (5, 7%).
The number of non-paracetamol DILI admissions was similar across the study period, but the proportion linked with herbal and dietary supplements increased from 2 of 11 (15%) during 2009-11 to 10 of 19 (47%) during 2018-20 (linear trend: P = 0.011). Despite higher median baseline model for end-stage liver disease (MELD) scores, 90-day transplant-free survival for patients with paracetamol-related DILI was higher than for patients with non-paracetamol DILI (86%; 95% CI, 79-93% v 71%; 95% CI, 60-82%) and herbal and dietary supplement-related cases (59%; 95% CI, 34-85%). MELD score was an independent predictor of poorer 90-day transplant-free survival in both paracetamol-related (per point increase: adjusted hazard ratio [aHR], 1.19; 95% CI, 1.09-3.74) and non-paracetamol DILI (aHR, 1.24; 95% CI, 1.14-1.36).
The authors concluded that, in our single centre study, the proportion of cases of people hospitalised with DILI linked with herbal and dietary supplements has increased since 2009. Ninety-day transplant-free survival for patients with non-paracetamol DILI, especially those with supplement-related DILI, is poorer than for those with paracetamol-related DILI.
A co-author of the paper, specialist transplant hepatologist Dr Ken Liu, was quoted in the Guardian saying he felt compelled to conduct the study because he was noticing more patients with liver injuries from drugs not typically associated with liver harm. “I was starting to see injury in patients admitted with liver injury after using bodybuilding supplements for males or weight loss supplements in females,” he said. “I just decided I better do a study on it to see if my hunch that more of these substances were causing these injuries was true.”
Liu and his colleagues said there needed to be more rigorous regulatory oversight for supplements and other alternative and natural therapies. They also noticed almost half the patients with supplement-induced severe liver injury had non-European ethnic backgrounds. Liu said more culturally appropriate community education about the risks of supplements was needed.
Dr Ken Harvey, public health physician and president of Friends of Science in Medicine, said it was important to note that Liu’s study only examined the most severe cases of supplement-induced liver harm and that the actual rate of harm was likely much higher. “The study only examines severe cases admitted to a specialised liver unit; they cannot be extrapolated to the overall incidence of complementary medicine associated liver injury in Australia,” Harvey said.
The Royal Australian College of General Practitioners, Choice, Friends of Science in Medicine and others have called for an educational statement on the pack and promotional material of medicines making traditional claims, for example saying “This product is based on traditional beliefs and not modern scientific evidence”.
“This was opposed by industry and the TGA,” Harvey said. “But is still needed.”
Pelargonium sidoides, a traditional medicinal plant native to South Africa, is one of the ornamental geraniums that is thought to be effective in treating URTIs. The plant seems to contain a large variety of phytochemicals, including amino acids, phenolic acids, α-hydroxy-acids, vitamins, polyphenols, flavonoids, coumarins, coumarins glucosides, coumarin sulphates and nucleotides. It is mostly used to treat the symptoms of acute bronchitis, common cold and acute rhinosinusitis.
The present study aimed to assess the effectiveness of the liquid herbal drug preparation from the root extracts of Pelargonium sidoides in improving symptoms of uncomplicated upper respiratory tract infections (URTIs). One hundred sixty-four patients with URTI were randomized and given either verum containing the root extracts of Pelargonium sidoides (n = 82) or a matching placebo (n = 82) in a single-blind manner for 7 days. The median total scores of all symptoms (TSS) showed a significant decreasing trend in the group treated with the root extracts derived from Pelargonium sidoides compared to the placebo group from day 0 to day 7 (TSS significantly decreased by 0.85 points in the root extract group compared to a decrease of 0.62 points, p = 0.018). “Cough frequency” showed a significant improvement from day 0 to day 3 (p = 0.023). There was also detected a significant recovery in “sneezing” on day 3 via Brunner-Langer model, and it was detected that the extract administration given in the first 24 h onset of the symptoms had provided a significant improvement in day 0 to day 3 (difference of TSS 0.18 point, p = 0.011).
The authors concluded that Pelargonium sidoides extracts are effective in relieving the symptom burden in the duration of the disease. It may be regarded as an alternative option for the management of URTIs.
These findings are less surprising than they may seem. Already in 2008, we published the following systematic review:
Objective: To critically assess the efficacy of Pelargonium sidoides for treating acute bronchitis.
Data sources: Systematic literature searches were performed in 5 electronic databases: (Medline (1950 – July 2007), Amed (1985 – July 2007), Embase (1974 – July 2007), CINAHL (1982 – July 2007), and The Cochrane Library (Issue 3, 2007) without language restrictions. Reference lists of retrieved articles were searched, and manufacturers contacted for published and unpublished materials.
Review methods: Study selection was done according to predefined criteria. All randomized clinical trials (RCTs) testing P. sidoides extracts (mono preparations) against placebo or standard treatment in patients with acute bronchitis and assessing clinically relevant outcomes were included. Two reviewers independently selected studies, extracted and validated relevant data. Methodological quality was evaluated using the Jadad score. Meta-analysis was performed using a fixed-effect model for continuous data, reported as weighted mean difference with 95% confidence intervals.
Results: Six RCTs met the inclusion criteria, of which 4 were suitable for statistical pooling. Methodological quality of most trials was good. One study compared an extract of P. sidoides, EPs 7630, against conventional non-antibiotic treatment (acetylcysteine); the other five studies tested EPs 7630 against placebo. All RCTs reported findings suggesting the effectiveness of P. sidoides in treating acute bronchitis. Meta-analysis of the four placebo-controlled RCTs suggested that EPs 7630 significantly reduced bronchitis symptom scores in patients with acute bronchitis by day 7. No serious adverse events were reported.
Conclusion: There is encouraging evidence from currently available data that P. sidoides is effective compared to placebo for patients with acute bronchitis.
Meanwhile, P.sidoides has been associated with liver damage, a fact that might dampen our enthusiasm for this remedy. Nevertheless, it seems to me that this plant merits further study.
On FACEBOOK I recently found this advertisement posted by ‘LifeCell Health’
Guys, weight loss starts at our gut. The reishi mushroom targets this key area of the body and promotes weight loss in a unique way, by changing our gut bacteria to digest food in a manner that improves weight loss and can even prevent weight gain. By combining 3 of the most researched mycological species on the planet, LifeCell Myco+ delivers a blend of weight loss mushrooms like no other: Improve gut health, speed up weight loss, enhance immune function, natural energy and more with our blend of Reishi, Turkey Tail, and Shiitake mushrooms. Each mushroom has been the subject of several in-vivo studies proving their efficacy when it comes to weight loss.
![]() Why Mushrooms Work.
Why Mushrooms Work.
![]() Reishi: Prevents weight gain by altering bacteria inside the digestive system
Reishi: Prevents weight gain by altering bacteria inside the digestive system
![]() Shiitake: Helps the body develop less fat by nourishing good gut bacteria.
Shiitake: Helps the body develop less fat by nourishing good gut bacteria.
![]() Turkey Tail: Reduces inflammation and helps prevent weight gain.
Turkey Tail: Reduces inflammation and helps prevent weight gain.
That sounded interesting, I thought, and I investigated a bit further. On the website of the firm, I found this text:
By combining 3 of the most researched mycological species on the planet, LifeCell Myco+ delivers an organic wellness formula unlike any other. Improve gut health, speed up weight loss, enhance immune function, natural energy and more with our blend of Reishi, Turkey Tail, and Shiitake mushrooms.
Keeping a healthy balance of beneficial bacteria in your gut is critical for maintaining a strong immune system. Your gut bacteria interact with immune cells and directly impact your immune response. Turkey tail mushrooms contain prebiotics, which help nourish these helpful bacteria. An 8-week study in 24 healthy people found that consuming 3,600 mg of PSP extracted from turkey tail mushrooms per day led to beneficial changes in gut bacteria and suppressed the growth of the possibly problematic E. coli and Shigella bacteria.
Next, I conducted a few Medline searches but was unable to find any trial data suggesting that any of the three mushrooms or their combination might reduce body weight. So, I wrote to the company:
Dear Madam/Sir
I am intrigued by your product MYCO +. Would you be kind enough to send me the studies showing that it can reduce body weight?
Many thanks
Edzard Ernst
What followed was a bizarre correspondence with several layers of administrators in the firm. They all said that I should discuss this with the next higher person. So, I asked myself up the hierarchy of LiveCell. The last email I received was this one:
Good morning Edzark,
Thank you for your email and I hope you are enjoying your day.
It is great to hear that you are interested in our LifeCell Myco. I have forwarded your request for additional information and once received I will be sure to forward the information to you.
What do I conclude from this experience?
Apart from being unable to get my name right, the people responsible at ‘LifeCell Health’ seem also not able to send me the evidence I asked for. This, I fear, means that there is no such evidence which means the claims are unsubstantiated. Scientifically, this might amount to misconduct; legally, it could be fraudulent.
But I am, of course, no lawyer and therefore leave it to others to address the legal issues.
PS
If anyone happens to know of some evidence, please let me know and I will correct my post accordingly.
Withania somnifera, commonly known as Ashwagandha, is a plant belonging to the family of Solanaceae. It is widely used in Ayurvedic medicine. The plant is promoted as an immunomodulator, anti-inflammatory, anti-stress, anti-Parkinson, anti-Alzheimer, cardioprotective, neural and physical health enhancer, neuro-defensive, anti-diabetic, aphrodisiac, memory-boosting, and ant-cancer remedy. It contains diverse phytoconstituents including alkaloids, steroids, flavonoids, phenolics, nitrogen-containing compounds, and trace elements.
But how much of the hype is supported by evidence? Unsurprisingly, there is a shortage of good clinical trials. Yet, during the last few years, a surprising number of reviews of the accumulating evidence have emerged:
- One review suggested that pre-clinical, as well as clinical studies, suggest the effectiveness of Withania somnifera (L.) against neurodegenerative disease.
- A further review suggested a potential role of W. somnifera in managing diabetes.
- A systematic review of 5 clinical trials found that W. somnifera extract improved performance on cognitive tasks, executive function, attention, and reaction time. It also appears to be well tolerated, with good adherence and minimal side effects.
- Another systematic review included 4 clinical trials and reported significant improvements in serum hormonal profile, oxidative biomarkers, and antioxidant vitamins in seminal plasma. No adverse effects were reported in infertile men taking W. somnifera treatment.
- Another review concluded that the root of the Ayurvedic drug W. somnifera (Aswagandha) appears to be a promising safe and effective traditional medicine for management of schizophrenia, chronic stress, insomnia, anxiety, memory/cognitive enhancement, obsessive-compulsive disorder, rheumatoid arthritis, type-2 diabetes and male infertility, and bears fertility promotion activity in females adaptogenic, growth promoter activity in children and as adjuvant for reduction of fatigue and improvement in quality of life among cancer patients undergoing chemotherapy.
- A systematic review of 13 RCTs found that Ashwagandha supplementation was more efficacious than placebo for improving variables related to physical performance in healthy men and women.
- Another systematic review concluded that Ashwagandha supplementation might improve the VO2max in athletes and non-athletes.
Impressed?
This certainly looks as though that this plant is worthy of further study. But I can never help feeling a bit skeptical when I hear of such a multitude of benefits without evidence for adverse effects (other than minor upset stomach, nausea, and drowsiness).
Ever since I published a post about the irresponsible and aggressive advertising campaign of LYMA (“the world’s 1st super-supplement”), I am pursued by them with emails informing me about the wonders of this supplement. Here is one I received recently:
Here at LYMA we are firm believers that optimal productivity depends on good quality sleep and your day is only as good as the previous night.
Suffering from bad sleep is debilitating whether it’s ourselves or we’re watching someone we love suffer, the search for good rest is something we’re all united in.
Energy levels, positive mindset and strong cognitive function all come from sleep, which is why we spent so long formulating the LYMA supplement. Our patented KSM-66® Ashwagandha is the highest-quality, zero toxicity, concentrated Ashwagandha root in the world. The hefty combination of purity and potency make it unrivalled in its ability to reduce inflammation, neutralise anxiety and promote deep, restful sleep, night after night.
Thousands of customers have told us that after years of bad sleep, they’re finally getting the rest they need and feeling transformed as a result. In fact, it’s one of the very first benefits most people notice. We’re happy to hear it.
And the knock-on effects of a good night’s sleep in how we feel, how we perform and our overall health are far reaching. Which is why we are so delighted to welcome Michael Grandner, world-renowned sleep expert and Director of the Behavioural Sleep Medicine Clinic, Arizona to the LYMA team.
Michael is one of the most cited sleep experts in the world and has himself published over 175 articles on issues relating to sleep and health. We plan on tapping into every area of his expertise to understand our own sleep habits and how we can all become the best at rest.
To introduce Michael to the LYMA community we’re hosting a seminar dedicated to understanding sleep on Tuesday 22nd June…
I was tempted to discard all this as rather pathetic advertising hype. But then I had second thoughts. This text does after all make several medical claims, and the question is: ARE THEY SUPPORTED BY EVIDENCE?
It claims that KSM-66® Ashwagandha:
- is the highest-quality, zero toxicity, concentrated Ashwagandha root in the world.
- That the hefty combination of purity and potency makes it unrivalled in its ability to reduce inflammation.
- That the product neutralises anxiety.
- That it promotes deep, restful sleep, night after night.
I ran a few searches to find out whether there is any sound evidence for any of these claims.
- There seem to be several supplements that contain,KSM-66® Ashwagandha’. The impression that LYMA is the only one is thus wrong. Zero toxicity must also be wrong; not even water has zero toxicity. In fact, epigastric pain/discomfort and loose stools were reported as most common (>5%); and giddiness, drowsiness, hallucinogenic, vertigo, nasal congestion (rhinitis), cough, cold, decreased appetite, nausea, constipation, dry mouth, hyperactivity, nocturnal cramps, blurring of vision, hyperacidity, skin rash and weight gain have all been associated with the herbal remedy. Moreover, if it is true that Ashwagandha stimulates the immune system, it might cause problems for people with autoimmune diseases.
- I found no compelling evidence from clinical trials to show that KSM-66® Ashwagandha reduces inflammatory conditions in humans.
- I found a study concluding that Ashwagandha given as an adjunct offered some potential advantages as a safe and effective adjunctive therapy to SSRIs in GAD. Yet, I found no compelling evidence from clinical trials to show that KSM-66® Ashwagandha as a single supplement reduces anxiety in otherwise healthy individuals.
- A 2021 study suggested that Ashwagandha root extract can improve sleep quality and can help in managing insomnia. Yet the authors cautioned that additional clinical trials are required to generalize the outcome.
So, what does that tell us?
It could mean that:
- My searches were not sufficiently thorough and that I have missed compelling evidence. If so, I would appreciate, if the LYMA promoters would show me their evidence so that I can assess it.
- The LYMA people are irresponsible and mislead the public with untenable claims.
I am looking forward to their response.
Due to polypharmacy and the rising popularity of so-called alternative medicines (SCAM), oncology patients are particularly at risk of drug-drug interactions (DDI) or herb-drug interactions (HDI). The aims of this study were to assess DDI and HDI in outpatients taking oral anticancer drugs.
All prescribed and non-prescribed medications, including SCAMs, were prospectively collected by hospital pharmacists during a structured interview with the patient. DDI and HDI were analyzed using four interaction software programs: Thériaque®, Drugs.com®, Hédrine, and Memorial Sloan Kettering Cancer Center (MSKCC) database. All detected interactions were characterized by severity, risk, and action mechanism. The need for pharmaceutical intervention to modify drug use was determined on a case-by-case basis.
A total of 294 patients were included, with a mean age of 67 years [55-79]. The median number of chronic drugs per patient was 8 [1-29] and 55% of patients used at least one SCAM. At least 1 interaction was found for 267 patients (90.8%): 263 (89.4%) with DDI, 68 (23.1%) with HDI, and 64 (21.7%) with both DDI and HDI. Only 13% of the DDI were found in Thériaque® and Drugs.com® databases, and 125 (2.5%) were reported with a similar level of risk on both databases. 104 HDI were identified with only 9.5% of the interactions found in both databases. 103 pharmaceutical interventions were performed, involving 61 patients (20.7%).
The authors concluded that potentially clinically relevant drug interactions were frequently identified in this study, showing that several databases and structured screening are required to detect more interactions and optimize medication safety.
These data imply that DDIs are more frequent than HDIs. This does, however, not tell us which are more important. One crucial difference between DDIs and HDIs is that the former are usually known to the oncology team who should thus be able to prevent them or deal with them appropriately; in contrast, HDIs are often not known to the oncology team because many patients fail to disclose the fact that they take herbal remedies. Some forget, some do not think of herbals as medicine, others may be worried about their physician’s reaction.
It follows that firstly, conventional healthcare practitioners should always ask about the usage of herbal remedies, and secondly, they need to be informed about which herbal remedy might interact with which drug. The first can easily be implemented into routine history-taking; the second is more problematic, not least because our knowledge about HDIs is still woefully incomplete. In view of this, it might often be wise to tell patients to stop taking herbal remedies while they are on prescription drugs.
A low intake of selenium has been associated with increased cardiovascular mortality in some epidemiological studies. This could be reduced by supplementation with selenium and coenzyme Q10. D-dimer, a fragment of fibrin mirroring fibrinolysis, is a biomarker of thromboembolism, increased inflammation, endothelial dysfunction and is associated with cardiovascular mortality in ischemic heart disease.
The objective of this trial was to examine the impact of selenium and coenzyme Q10 on the level of D-dimer, and its relationship to cardiovascular mortality. D-dimer was measured in 213 individuals at the start and after 48 months of a randomised double-blind placebo-controlled trial with selenium yeast (200 µg/day) and coenzyme Q10 (200 mg/day) (n = 106) or placebo (n = 107). The follow-up time was 4.9 years.
All included individuals were low in selenium (mean 67 μg/L, SD 16.8). The differences in D-dimer concentration were evaluated by the use of T-tests, repeated measures of variance, and ANCOVA analyses. At the end, a significantly lower D-dimer concentration was observed in the active treatment group in comparison with those on placebo (p = 0.006). Although D-dimer values at baseline were weakly associated with high-sensitive CRP, while being more strongly associated with soluble tumour necrosis factor receptor 1 and sP-selectin, controlling for these in the analysis there was an independent effect on D-dimer.
In participants with a D-dimer level above median at baseline, the supplementation resulted in significantly lower cardiovascular mortality compared to those on placebo (p = 0.014). All results were validated with a persisting significant difference between the two groups.
The authors concluded that supplementation with selenium and coenzyme Q10 in a group of elderly low in selenium and coenzyme Q10 prevented an increase in D-dimer and reduced the risk of cardiovascular mortality in comparison with the placebo group. The obtained results also illustrate important associations between inflammation, endothelial function and cardiovascular risk.
These results are interesting and potentially important. The authors agree that their study is not fully conclusive: “Even if the size of the study population is small, we regard the results as being interesting from a scientific point of view, and for hypothesis-generating. The included participants represented a relatively narrow age stratum, so it is not possible to extrapolate the results to other age groups without uncertainty. Finally, as the evaluated population consisted of Caucasians who were low in selenium and coenzyme Q10, it is not necessarily true that the obtained results could be extrapolated to another population.” It might furthermore be of interest to note that part of the analysis cost was supported by grants from Pharma Nord Aps, Denmark, the County Council of Östergötland, Linköping University.
What is needed next, I think, are independent replications. Also of interest would be to determine whether the effects are due to the selenium, or the Q10, or both. And finally, one must caution consumers to not overdose on selenium which could have a host of negative effects on health.
