causation
An epidemiological study from the US just published in the BMJ concluded that “the mortality gap in Republican voting counties compared with Democratic voting counties has grown over time, especially for white populations, and that gap began to widen after 2008.”
In a BMJ editorial, Steven Woolf comments on the study and provides further evidence on how politics influence health in the US. Here are his concluding two paragraphs:
Political influence on US mortality rates became overt during the covid-19 pandemic, when public health policies, controlled by states, were heavily influenced by party affiliation. Republican politicians, often seeking to appeal to President Trump and his supporters, challenged scientific evidence and opposed enforcement of vaccinations and safety measures such as masking. A macabre natural experiment occurred in 2021, a year marked by the convergence of vaccine availability and contagious variants that threatened unvaccinated populations: states led by governors who promoted vaccination and mandated pandemic control measures experienced much lower death rates than the “control” group, consisting of conservative states with lax policies and large unvaccinated populations. This behavior could explain why US mortality rates associated with covid-19 were so catastrophic, vastly exceeding losses in other high income countries.
Observers of health trends in the US should keep their eye on state governments, where tectonic shifts in policy are occurring. While gridlock in Washington, DC incapacitates the federal government, Republican leaders in dozens of state capitols are passing laws to undermine health and safety regulations, ban abortion, limit LGBT+ rights, and implement more conservative policies on voting, school curriculums, and climate policy. To understand the implications for population health, researchers must break with custom; although scientific literature has traditionally avoided discussing politics, the growing influence of partisan affiliation on policies affecting health makes this covariate an increasingly important subject of study.
_____________________
What has this to do with so-called alternative medicine (SCAM)?
Not a lot.
Except, of course, that Trump has been quite sympathetic to both quackery and quacks (see, for instance, here and here). Moreover, the embarrassing Dr. Oz, America’s charlatan-in-chief, is now a Republican candidate for the US senate. And the creation of the NHI office for alternative medicine, currently called NCCIH, was the idea of the Republican senator, Tom Harkin.
I think we get the drift: on the US political level, SCAM seems to be a right-wing thing.
Am I claiming that SCAM is the cause of the higher mortality in Republican counties?
No.
Do I feel that both are related to irresponsible attitudes towards healthcare issues?
Yes.
In India, the homeopathic remedy, Arsenicum Album 30C (prepared from arsenic trioxide) is widely prescribed and publicly supplied to adults and children for preventing COVID infections. Inorganic arsenic, known as the “king of poisons” is a highly toxic substance with the potential to cause acute as well as chronic injury to multiple organ systems, mainly skin, lung, liver, and kidneys.
Indian researchers present three cases of acute liver injury, leading to the death of one patient with underlying non-alcoholic steatohepatitis (NASH) cirrhosis, after consumption of the homeopathic remedy AA30 for COVID-19 prevention.
Case one
A 70-year-old man with compensated non-alcoholic steatohepatitis (NASH)-related cirrhosis and diabetes mellitus consumed the homeopathic IB AA30 as prescribed for 12 weeks prior to the onset of symptoms. He presented with jaundice and abdominal distension within four weeks after the onset of loss of appetite and well-being. The patient was not on any other hepatotoxic agents, over-the-counter medications, or herbal and dietary supplements. Investigations revealed the presence of conjugated hyperbilirubinemia, ascites, and abnormal coagulation, suggestive of acute-on-chronic liver failure (ACLF). Further investigations to identify known causes of acute deterioration of underlying cirrhosis were performed, including a transjugular liver biopsy. All competing causes for acute liver injury were meticulously ruled out. These included infections-tests for immunoglobulin M (IgM) for viral hepatitis A and E; hepatitis B surface antigen and IgM antibody to hepatitis B core antigen; nucleic acid tests via polymerase chain reaction for hepatitis C; IgM for herpes zoster and herpes simplex, cytomegalovirus, parvovirus, Epstein-Barr virus. Complete auto-antibody testing for autoimmune hepatitis (AIH) was negative. The Roussel Uclaf Causality Assessment (RUCAM) demonstrated “probable” (score 7) drug-induced liver injury (DILI) and simplified AIH score was less than 5, revealing the cause of acute liver injury leading to ACLF as the homeopathic remedy, AA30. The liver biopsy revealed multiacinar hepatocyte necrosis, lymphocytic, neutrophilic, and eosinophilic inflammation in the absence of interface hepatitis, which were predominantly portal-based in the background of cirrhosis, suggestive of DILI. Analysis of drugs consumed could not be performed in view of inadequate sample availability. The patient and family consented to arsenic analysis in nail and hair samples which revealed extremely high levels of the heavy metal, supportive of arsenic toxicity and associated liver injury in the patient. Evaluation of hair and hair samples of two family members (below detection limits, method detection limit being 0.1 mg/kg), staying in the same household did not reveal levels signifying cluster arsenic poisoning from water or soil sources. The patient succumbed to complications related to ACLF, nine months after the initial diagnosis.
Case two
A 68-year-old male with systemic hypertension controlled on telmisartan who ingested AA30 as prescribed for four weeks prior to the onset of symptoms. There was no associated jaundice or cholestatic symptoms, but liver tests revealed acute hepatitis with an elevation of liver enzymes. The patient was not on any other hepatotoxic agents, over-the-counter medications, or herbal and dietary supplements. Further investigations did not reveal the presence of underlying chronic liver disease or portal hypertension. All competing causes for acute liver injury were meticulously ruled out similar to the extensive workup that was done in case one. The RUCAM demonstrated “probable” (score 8) DILI and simplified AIH score was less than 5, revealing the cause of acute non-icteric hepatitis as the homeopathic remedy, AA30. The liver biopsy revealed perivenular hepatocyte necrosis, with predominantly portal-based mixed cellular inflammation consisting of plasma cells, eosinophils, lymphocytes, and scattered neutrophils. Additionally, ballooning of hepatocytes was marked with scattered rosettes and moderate interphase hepatitis in the presence of mild portal and sinusoidal fibrosis suggestive of DILI. Acute hepatitis resolved after drug withdrawal and finite course of steroids within three months, without any recurrence on follow-up.
Case three
A 48-year-old overweight woman consumed homeopathic AA30 pills as COVID-19 preventive for one week prior to the onset of her symptoms of cholestatic jaundice. Prior to the development of jaundice, she had nonspecific gastrointestinal symptoms such as nausea and progressive loss of appetite. Liver tests revealed conjugated hyperbilirubinemia with highly raised liver enzymes. The patient was not on any other hepatotoxic prescription drugs, over-the-counter medications, or herbal and dietary supplements. Further investigations did not reveal the presence of underlying chronic liver disease or portal hypertension. All competing causes for acute liver injury were meticulously ruled out similar to the extensive workup that was done in case one. The RUCAM demonstrated “probable” (score 7) DILI and simplified AIH score was less than 5, revealing the cause of acute cholestatic hepatitis as the homeopathic remedy, AA30. The liver biopsy revealed spotty, focal hepatocyte necrosis, with predominantly portal-based neutrophilic and eosinophil-rich inflammation, moderate steatosis, and mild interface hepatitis with underlying mild perisinusoidal fibrosis, suggestive of DILI. The acute cholestatic hepatitis resolved after drug withdrawal and a finite course of steroids within six months, without any recurrence on follow-up.
The chemical analysis and toxicology (inductively coupled optical emission spectroscopy and triple-quadrupole gas chromatography with tandem mass spectroscopy method) on two sets of AA30 samples retrieved from case three revealed D-mannose, melezitose, and arsenic respectively, demonstrating batch-to-batch variation due to poor manufacturing practices.
The authors draw the following conclusions: Health regulatory authorities, physicians, general and patient population must be aware of the potential harms associated with the large-scale promotion of untested, alternative medical systems during a medical emergency so as to prevent an “epidemic” of avoidable DILI within the ongoing pandemic. Even though ultra-diluted homeopathic remedies, found ineffective as shown in large-scale meta-analysis, are considered safe for use due to the absence of any active compound beyond 12C dilution. Nonetheless, poor manufacturing practices, use of concentrated tincture formulations, and adulteration and contamination of homeopathic remedies can still pose considerable toxicity in predisposed persons. From a scientific and evidence-based standpoint, it is imperative that the general population and at-risk persons understand that vaccination, and not untested, misleading IBs, remains the best available armamentarium against COVID-19 which helps in fighting back the pandemic.
Nausea and vomiting are common symptoms of patients with advanced cancer. While there is some evidence for acupuncture point stimulation in the treatment of these symptoms for patients having anticancer treatment, there is little for when they are not related to such treatment.
This study aimed to determine whether acupressure at the pericardium 6 sites can help treat nausea and vomiting suffered by palliative care patients with advanced cancer. The researchers conducted a double-blind randomized controlled trial-active versus placebo acupressure wristbands. In-patients with advanced cancer in two specialist palliative care units who fitted either or both of the following criteria were approached: nausea that was at least of moderate severity; vomiting daily on average for the prior 3 days.
A total of 57 patients were randomized to have either active or placebo acupressure wristbands. There was no difference in any of the outcome measures between the two groups:
- change from the baseline number of vomits;
- Visual Analogue Scale for ‘did acupressure wristbands help you to feel better?’;
- the total number of doses of antiemetic medication;
- the need for escalation of antiemetics.
The authors concluded that, in contrast to a previously published feasibility study, active acupressure wristbands were no better than placebo for specialist palliative care in patients with advanced cancer and nausea and vomiting.
When the research into acupuncture for nausea and vomiting began some 20 years ago, the evidence turned out to be encouraging. Later, as the studies became more and more rigorous, many trials failed to confirm the initial findings. Today, the totality of the evidence is far less convincing than it seemed years ago.
This is a phenomenon that can be observed not just in acupuncture research but in many types of treatment:
- Initially, over-enthusiastic researchers become victims of their own optimism.
- These investigators are less into testing hypotheses than into confirming their own wishful thinking.
- Thus, several positive trials emerge.
- These, however, turn out to be methodologically flawed.
- Eventually, the subject might be picked up by real scientists who truly test hypotheses.
- More and more negative studies thus emerge.
- Depending on how many flawed studies were initially published and how critical the authors of systematic reviews are, it can take years until the totality of the evidence depicts the true picture which discloses the initial findings as false-positive.
The message is, I think, clear: poor quality studies have the potential to mislead us for many years. Eventually, however, the self-cleansing ability of science should generate the truth about the value of any treatment. In other words:
poor-quality science is not just useless, it causes long-term harm
and
critical thinking prevents harm
As promised, I would like to correct the errors in my previous assessment of this paper. To remind everyone:
This systematic review evaluated individualized homeopathy as a treatment for children with attention deficit and hyperactivity disorder (ADHD) when compared to placebo or usual care alone.
Thirty-seven online sources were searched up to March 2021. Studies investigating the effects of individualized homeopathy against any control in ADHD were eligible. Data were extracted to a predefined excel sheet independently by two reviewers.
Six studies were analyzed:
- 5 were RCTs
- 2 were controlled against standard treatments;
- 4 were placebo-controlled and double-blinded.
The meta-analysis revealed a significant effect size across studies of Hedges’ g = 0.542 (95% CI 0.311-0.772; z = 4,61; p < 0.001) against any control and of g = 0.605 (95% CI 0.05-1.16; z = 2.16, p = 0.03) against placebo. The effect estimations are based on studies with an average sample size of 52 participants.
The authors concluded that individualized homeopathy showed a clinically relevant and statistically robust effect in the treatment of ADHD.
_______________________________
Now that I was able to access the full papers, I would like to offer a thorough analysis.
To get included in the review, primary studies had to be:
- Published after 1980,
- Investigating an individualized homeopathic intervention in childhood ADHD,
- Comparing the intervention to a control condition (placebo, standard care or treatment as usual, both of which are referred to as “active control”) in a randomized or non-randomized parallel-group study
design with one or more arms.
Six studies were included:
- Fibert, P., Peasgood, T. & Relton, C. Rethinking ADHD intervention trials: feasibility testing of two treatments and a methodology. Eur. J. Pediatr. 178, 983–993 (2019). – DOI
- Fibert, P., Relton, C., Heirs, M. & Bowden, D. A comparative consecutive case series of 20 children with a diagnosis of ADHD receiving homeopathic treatment, compared with 10 children receiving usual care. Homeopathy 105, 194–201 (2016). – DOI
- Jacobs, J., Williams, A. L., Girard, C., Njike, V. Y. & Katz, D. Homeopathy for attention-deficit/hyperactivity disorder: a pilot randomized-controlled trial. J. Altern. Complement. Med. 11, 799–806 (2005). – DOI
- Jones, M. The efficacy of homoeopathic simillimum in the treatment of attention-deficit/hyperactivity disorder (AD/HD) in schoolgoing children aged 6-11 years. https://openscholar.dut.ac.za/bitstream/10321/534/1/Jones_2009.pdf (2009).
- Frei, H. et al. Homeopathic treatment of children with attention deficit hyperactivity disorder: a randomised, double blind, placebo controlled crossover trial. Eur. J. Pediatr. 164, 758–767 (2005). – DOI
- Oberai, P. et al. Homoeopathic management of attention deficit hyperactivity disorder: a randomised placebo-controlled pilot trial. Indian J. Res. Homoeopathy 7, 158–167 (2013).
Exclusion criteria were:
- Homeopathic intervention not individualized,
- Serious methodological flaws, such as incidental unblinding, failure to report important data, or insufficient data for meta-analysis.
One study was excluded:
- Lamont, J. Homoeopathic treatment of attention deficit hyperactivity disorder. Br. Homeopathic J. 86, 196–200 (1997). – DOI
I will first make several points about Walach’s systematic review itself and then have a look at the primary studies that it included. Finally, I will try to draw some conclusions.
The review authors state in their introduction that “beneficial effects of this intervention [homeopathy] have been shown for various kinds of medical conditions, including child diarrhea, supportive care in cancer, fibromyalgia, or ADHD.” In other words, already in the introduction, they disclose their strong pro-homeopathy bias; it would, of course, not be difficult to find investigations that contradict their optimism.
Despite the stated inclusion/exclusion criteria, the authors did include the Frei-study that did not follow a parallel-group design (see also below).
The authors included two active-controlled studies both of which did not report the type of treatment received by the control group. In other words, these trials failed to report important data which was a stated exclusion criterium (see below).
In their discussion section, the authors state that “all included studies employed individualized homeopathy and were of comparable, solid quality, hence a lack of methodological rigor is unlikely the reason for the difference between homeopathy and controls…” This, I think, is grossly misleading; even according to the authors’ own assessments, one study was deemed to have a high risk of bias and in two studies the risk of bias was “unclear”.
The overall positive effect of homeopathy demonstrated by the review was determined almost exclusively by the study of Oberai et al (p-value = 0.000). In fact, the studies by Jones and by Jacobs were negative, and the one by Frei was borderline positive with a p-value of 0.46. The authors address this crucial issue repeatedly and claim that excluding Oberai et al would still generate an overall positive meta-analytic result. Yet, they do not mention that the overall result would no longer be clinically relevant.
Looking at the included primary studies, I should make the following points:
- The two Filbert studies, as mentioned, failed to report important data and should, according to the stated exclusion criteria, not have been included.
- The study by Jacobs was a pilot study and generated negative findings.
- The study by Jones is a non-peer-reviewed thesis. In my view, it should never have been included.
- The study by Frei was a cross-over trial. According to the exclusion/inclusion criteria of the authors, it should not have been included.
- The study by Oberai et al is the trial that has by far the largest effect size and thus is the driver of the overall result of the review. It is therefore important to have a closer look at it.
Here is the abstract:
Objective: To evaluate the usefulness of individualised homoeopathic medicines in treatment of Attention Deficit Hyperactivity Disorder (ADHD).
Design: Randomised placebo-controlled single-blind pilot trial.
Setting: Central Research Institute (Homoeopathy), Kottayam, Kerala, India from June 2009 to November 2011.
Participants: Children aged 6-15 years meeting the Diagnostic Statistical Manual of mental disorders (DSM-IV) criteria for ADHD.
Interventions: A total of 61 patients (Homoeopathy = 30, placebo = 31) were randomised to receive either individualised homoeopathic medicine in fifty millesimal (LM) potency or placebo for a period of one year.
Outcome measures: Conner’s Parent Rating Scale-Revised: Short (CPRS-R (S)), Clinical Global Impression-Severity Scale (CGI-SS), Clinical Global Impression- Improvement Scale (CGI-IS) and Academic performance.
Results: A total of 54 patients (homoeopathy = 27, placebo = 27) were analysed under modified intention to treat (ITT). All patients in homoeopathy group showed better outcome in baseline adjusted General Linear Model (GLM) repeated measures ANCOVA for oppositional, cognition problems, hyperactivity and ADHD Index (domains of CPRS-R (S)) and CGI-IS at T3, T6, T9 and T12 (P = 0.0001). The mean baseline-adjusted treatment difference between groups at month 12 from baseline for all individual outcome measures favoured homoeopathy group; Oppositional (−16.4, 95% CI – 20.5 to − 12.2, P = 0.0001), Cognition problems (−15.5, 95% CI − 19.2 to − 11.8, P = 0.0001), Hyperactivity (−20.6, 95% CI − 25.6 to − 15.4, P = 0.0001), ADHD I (−15.6, 95% CI − 19.5 to − 11.6, P = 0.0001), Academic performance 14.4%, 95% CI 8.3 to 20.5, P = 0.0001), CGISS (−1.6, 95% CI − 1.9 to − 1.2, P = 0.0001), CGIIS (−1.6, 95% CI − 2.3 to -0.9, P = 0.0001).
Conclusion: This pilot study provides evidence to support the therapeutic effects of individualised homoeopathic medicines in ADHD children. However, the results need to be validated in multi-center randomised double-blind placebo-controlled clinical trial.
Here are a few points of concern related to the Oberai et al:
- The trial was a mere pilot study.
- Despite the fact that it is now 9 years old, the authors never published a definitive trial.
- The study was published in an obscure journal that is not Medline-listed.
- The study is very poorly reported.
- It is unclear how the diagnosis of ADHD for including the patients was verified.
- The control patients were treated for one year with a placebo and no other therapies. In my view, this is not ethical.
- The method of randomization is unclear.
- The authors state that acute symptoms were treated throughout the study period with homeopathy, even in the control group. This seems odd and defies the principle of a placebo-controlled trial.
- The authors state that only the patients were blind, not the investigators. This opens the door wide for all sorts of biases. It is, for example, likely that it also de-blinded the patients (the verum could be adjusted and changed, while the placebo remained constant).
All in all, this paper is of poor quality, Its findings are far from trustworthy and were not meant to be definitive. According to the following exclusion criteria, it should have been excluded:
- It had several serious methodological flaws.
- It did not blind the investigators.
- It is likely that patients were de-blinded.
- It failed to report important data.
So, why did Walach and his co-authors include it?
Could it be because, without the Oberai-study, the overall findings of the review would at best have turned out to be borderline significant and not clinically relevant?
I came across an interesting case report recently published in an Austrian magazine. Here is my translation for non-German speakers:
A 42-year-old woman from Vienna has suffered from endometriosis since the age of 13. But it was only 8 years later that she found out what made the first two days of her menstruation so unbearable. She was not allowed to take painkillers to help herself during all that time. Her parents listened to medical “gurus” who distrusted conventional medicine.
“I grew up in a household where almost all illnesses were treated with homeopathy,” she wrote on Twitter. That’s exactly what became the IT expert’s undoing. In a recent interview, she looked back bitterly: “All infections and illnesses were treated with Bach flower remedies or homeopathics. Only in case of accidents or broken bones did my parents drive me to the hospital.” Her father suffered from an auto-immune disease. Because conventional medicine could not help him, he tried alternative approaches. “My parents slowly drifted more and more into this scene. At some point, they stopped listening to ‘normal’ doctors. It went downhill from there.”
As a girl, the Viennese had little chance of standing up to her parents’ “whisperers,” as she calls their esoteric advice. “When I got my period, I was in the worst pain. I fainted every month, even falling off my chair when I did it, once even at school. I vomited until I was so exhausted that I fell asleep.”
She begged her family to finally be allowed to consult a gynecologist. But he didn’t take the teenager seriously at the time and simply wanted to prescribe her the pill without a thorough examination. “I then went to my parents’ homeopathic ‘pill pusher’, who gave me homeopathics against my complaints. I wasn’t allowed to take painkillers because they ‘damage the liver’.” The guru persuaded the young woman that her health problems were her fault. “He said I just didn’t accept myself as a woman and that’s why I was in pain. I thought for a long time that I was just not strong and good enough.”
It wasn’t until she was already in her early 20s that her then-boyfriend took her to a gynecologist who finally took her condition seriously. “The ultrasound showed that I had quite a few cysts in my abdomen.” The diagnosis was also finally certain: she was now officially suffering from endometriosis. She was given the right medicine, and most of the endometriotic growths regressed. But a cyst had wrapped itself tightly around her right ovary, damaging it irrevocably over the years. It had died. “Homeopathy cost me my ovary,” the Viennese woman laments.
The fact that she nevertheless was able to become the mother of two children is thanks to her other ovary, which fortunately remained intact. But the feeling of having been treated wrongly, or not treated at all, for such a long time makes her angry. “I don’t blame my parents today. They have apologized and found their own way out of the gurus’ world of thought and out of the scene,” she emphasizes. “But I blame the people who pretend to be able to cure the majority of all diseases with homeopathy. Yet most of the time they can’t even find the right diagnosis and just give patients some stuff that has no side effects.” She now calls for an end to homeopathy.
_________________________
How many times have I said it?
His remedy might be risk-free, but the homeopath certainly isn’t!
Prof. Fabricio Benedetti is one of the world’s leading experts in the study of placebo effects. I have mentioned his excellent work before, for instance, here where he cautioned that quackery has today one more weapon on its side, which is paradoxically represented by the hard science–supported placebo mechanisms. Now he has expressed his concerns even more clearly in an article entitled “Alternative and natural medicine quackery is on the rise. Here’s why the placebo effect is part of the problem”. Here are a few excerpts from this excellent paper:
For several decades now, many scientists, including me, have been working hard to reveal the full power and scope of the placebo effect — the amazing ability of a simple sugar pill or other non-pharmaceutical “fake intervention” to improve someone’s quality of life. This research has been crucial to giving scientific credibility to a powerful psychological effect. But the advances of science have also backfired, spawning an alternative industry that preys on the vulnerable…
All this means that some alternative medicines can indeed have positive outcomes for patients, though not necessarily through the mechanisms that the therapy’s inventors supposed, but rather through a placebo effect. This holds true for treatments ranging from strange talismans to acupuncture — studies have shown that pain relief is about the same for patients receiving true acupuncture with needles, for example, as for those receiving sham acupuncture with trick needles.
The scientific advances in understanding placebo are fascinating. But one unfortunate outcome of all this work is that profit-seeking companies and individuals now have a new weapon: It is no longer necessary to demonstrate the effectiveness of their proposed therapies; it is enough to assert that these work because of the placebo effect. I receive myriad eccentric proposals for new therapies, ranging from talismans and concoctions to mascots and weird rituals. Their inventors claim that these are capable of inducing substantial health benefits and often seek my endorsement. These proposals have stepped up sharply in recent years. Sadly, the science of the placebo effect is fueling this new breed of pseudoscience…
So, if a salesperson says: “This concoction (or ritual or talisman) will reduce your pain,” it is not necessarily a lie, as the placebo effect may indeed stimulate pain-relieving circuits in the brain. But anyone could truthfully use these words, within limits.
These marketers often overstate the size of the possible response, claim to provide a “cure” rather than pain relief or incorrectly suggest that only their own expensive products will have this effect. Even worse, they may present the products as an alternative to more effective traditional medications for serious conditions such as cancer. In other words, they prey on the vulnerable by making undeliverable promises, purportedly backed by the science of placebo.
Even if taking a placebo can reduce symptoms such as pain, this isn’t always the best course of action. An apparently trivial pain may, for example, be the first sign of something far more serious. Treating the pain alone may prevent diagnosis by a physician or delay important medical treatments…
…Education, communication and honesty are the best friends of medical practice. Patients and health care professionals deserve to know what placebos can and cannot do.
The research and medical communities must be more transparent about the efficacy of many conventional pharmacological and nonpharmacological treatments, by acknowledging that some of them are useful whereas some others are not. Many over-the-counter products have doubtful efficacy, for example. Honesty will boost patients’ trust and confidence in medicine, which are the best antidotes to quackery.
BRAVO PROF BENEDETTI!
Two million people in UK are estimated to be currently suffering from long COVID, says the Office for National Statistics. Fatigue continues to be the most common symptom – experienced by 55% of those with self-reported long COVID – followed by 32% with shortness of breath, 23% with a cough, and 23% with muscle ache. The problem is only going to increase in the near future. Thus, many people are frantically looking for an effective therapy. Practitioners of so-called alternative medicine (SCAM) are no exception.
This study aimed to evaluate the potential for inhalation of essential oils to improve energy levels among otherwise healthy female survivors of acute COVID-19 who experience a lack of energy more than five months after recovery.
This was a randomized double-blind, placebo-controlled trial to evaluate the potential for inhalation of Longevity™, a proprietary essential oil blend manufactured by Young Living Essential Oils (Lehi, Utah, USA), on energy levels among female survivors of COVID-19 who continue to experience fatigue more than 5 months recovery from the acute infection. Forty women were randomized to two groups: intervention and placebo. The placebo product contained an inert, odorless fractionated coconut oil. Both groups inhaled the assigned product twice daily for fourteen consecutive days. Fatigue scores were measured using the Multidimensional Fatigue Symptom Inventory (MFSI). Secondary outcomes included scores on each of the MFSI’s ten subscales.
Individuals who inhaled the essential oil blend for 2 weeks had significantly lower fatigue scores after controlling for baseline scores, employment status, BMI, olfactory function, and time since diagnosis, with a large effect size (F (1,39) = 6.15, p = .020, partial eta squared = 0.198). Subscale analysis identified subscales of vigor, as well as global, behavioral, general, and mental fatigue as benefiting from the intervention. This study provides evidence that a proprietary aromatherapy blend can significantly improve energy levels among women who are experiencing fatigue after recovering from COVID-19.
The authors concluded that the use of aromatherapy with Longevity™ essential oil blend to boost energy levels in women who have recovered from COVID-19 provides a novel, non-invasive approach to improving quality of life in this population. This intervention is particularly beneficial for global and mental fatigue, as well as vigor. Other subdomains may experience improvements to energy levels with a smaller effect size; future studies should be conducted to explore this potential.
This trial was funded by Young Living Essential Oils. Perhaps, this explains why there is no mention of the elephant in the room: the trial was not blind! Participants in the verum group knew that they received aromatherapy. Likewise, participants in the placebo group knew that they received the placebo.
Could this fact have influenced the outcome? Certainly!
Could the trial have been designed better? Certainly!
All the investigators needed to do is to use a nice-smelling oil that, according to aromatherapists, does not boost energy, as the placebo.
As it stands, we have no idea whether the authors’ assumption that the verum oil caused the effect is true.
Pity!
Or maybe not?
Perhaps Young Living Essential Oils, the sponsor of the study and producer of the oil never wanted to know the truth. Maybe they are happy to abuse science as a marketing tool?
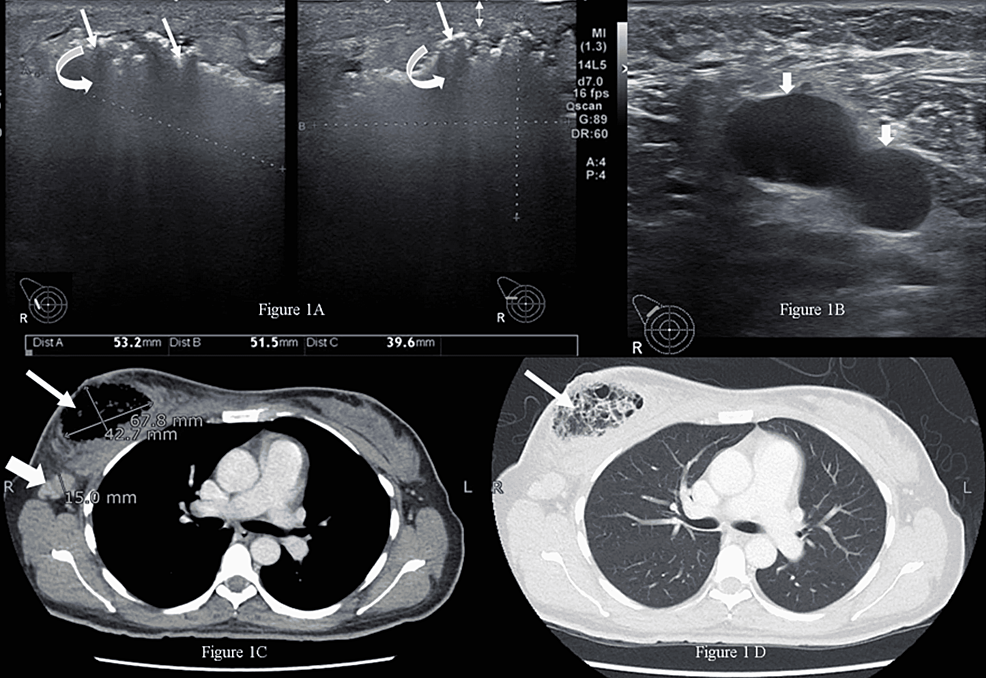
Necrotizing infection (NI) of the breast associated with underlying malignancy is a rare phenomenon characterized by necrosis of breast parenchyma. It can cause a delay in diagnosis and even lead to sepsis. Researchers from the Aga Khan University Hospital in Karachi, PAK, present a case of a 42-year-old woman with NI of the right breast, while on homeopathic treatment for a right breast lump for six months. Tissue culture showed a polymicrobial infection and histopathology established the diagnosis of breast carcinoma. After treating the NI, her breast cancer was managed as per standard guidelines.
The married, nulliparous, diabetic, hypertensive patient was a non-smoker and presented to the emergency room with complaints of fever, severe pain, and foul-smelling bloody discharge from her right breast for two weeks. She had a history of a right breast lump for six months, for which she had been taking oral homeopathic remedies, the names of which were not recorded. On examination, she had a blood pressure of 132/76 mmHg, a pulse of 84 bpm, a temperature of 99 °F, and a respiratory rate of 14 breaths per minute. The right breast was tender and hard, with a 4 x 3-cm necrotic skin patch on the upper half with bleeding and a palpable right axillary lymph node. The rest of the examination was unremarkable.
The patient was advised to undergo a metastatic workup in the emergency room, which included a contrast-enhanced CT (CECT) of the chest, abdomen, and pelvis and a bone scan. The CT confirmed the presence of an air-filled cavity in the right breast with thin septations and enlarged right axillary lymph nodes; however, there was no enhancing mass to suggest neoplasm in either breast. The CT and bone scans were negative for metastasis. The presence of severely tender breast on clinical examination and air within the breast on ultrasound suggested the possibility of NI, which warranted an early surgical intervention to prevent impending sepsis.
Microscopic examination of the debrided tissue revealed an invasive breast carcinoma of no special type [invasive ductal carcinoma (IDC), NST grade III] along with extensive necrosis and dense acute and chronic inflammation. The right axillary node biopsy was positive for nodal metastasis, and the patient was staged as cT4N1MO. A tissue culture showed a few colonies of Staphylococcus aureus and Enterococcus species suggestive of NI. After a discussion at a multidisciplinary tumor board meeting, the patient underwent a right modified radical mastectomy. Her postoperative course was unremarkable.
____________________
I have said it often but I am afraid I need to say it again: the homeopathic remedy might be harmless, but that does not mean that homeopathy is not dangerous.
Naprapathy is an odd variation of chiropractic. To be precise, it has been defined as a system of specific examination, diagnostics, manual treatment, and rehabilitation of pain and dysfunction in the neuromusculoskeletal system. It is aimed at restoring the function of the connective tissue, muscle- and neural tissues within or surrounding the spine and other joints. The evidence that it works is wafer-thin. Therefore rigorous studies are of interest.
The aim of this study was to evaluate the cost-effectiveness of manual therapy compared with advice to stay active for working-age persons with nonspecific back and/or neck pain.
The two interventions were:
- a maximum of 6 manual therapy sessions within 6 weeks, including spinal manipulation/mobilization, massage, and stretching, performed by a naprapath (index group),
- information from a physician on the importance to stay active and on how to cope with pain, according to evidence-based advice, on 2 occasions within 3 weeks (control group).
A cost-effectiveness analysis with a societal perspective was performed alongside a randomized controlled trial including 409 persons followed for one year, in 2005. The outcomes were health-related Quality of Life (QoL) encoded from the SF-36 and pain intensity. Direct and indirect costs were calculated based on intervention and medication costs and sickness absence data. An incremental cost per health-related QoL was calculated, and sensitivity analyses were performed.
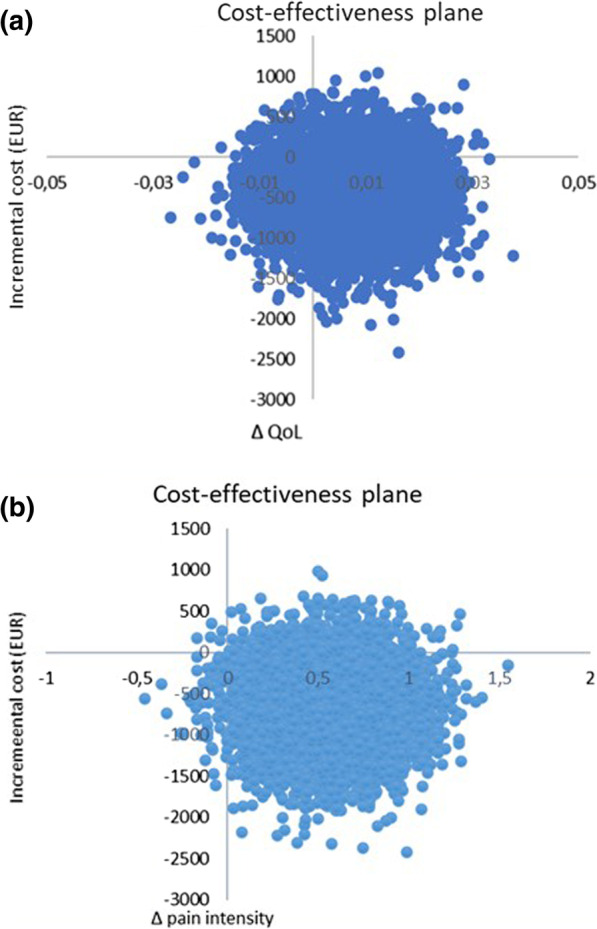
The difference in QoL gains was 0.007 (95% CI – 0.010 to 0.023) and the mean improvement in pain intensity was 0.6 (95% CI 0.068-1.065) in favor of manual therapy after one year. Concerning the QoL outcome, the differences in mean cost per person were estimated at – 437 EUR (95% CI – 1302 to 371) and for the pain outcome the difference was – 635 EUR (95% CI – 1587 to 246) in favor of manual therapy. The results indicate that manual therapy achieves better outcomes at lower costs compared with advice to stay active. The sensitivity analyses were consistent with the main results.
Cost-effectiveness plane using bootstrapped incremental cost-effectiveness ratios for QoL and pain intensity outcomes
The authors concluded that these results indicate that manual therapy for nonspecific back and/or neck pain is slightly less costly and more beneficial than advice to stay active for this sample of working age persons. Since manual therapy treatment is at least as cost-effective as evidence-based advice from a physician, it may be recommended for neck and low back pain. Further health economic studies that may confirm those findings are warranted.
This is an interesting and well-conducted study. The differences between the groups seem small and of doubtful relevance. The authors acknowledge this fact by stating: “together with the clinical results from previously published studies on the same population the results suggest that manual therapy may be as cost-effective a treatment as evidence-based advice from a physician, for back and neck pain”. Moreover, the data do not convince me that the treatment per se was effective; it might have been the non-specific effects of touch and attention.
I have said it before: there is currently no optimal treatment for neck and back pain. Therefore, the findings even of rigorous cost-effectiveness studies will only generate lukewarm results.
Osteopathic visceral manipulation (VM) is a bizarre so-called alternative medicine (SCAM) that has been featured on this blog with some regularity, e.g.:
- Osteopathic visceral manipulation: a new study fails to convince anyone
- Visceral manipulation…you couldn’t make it up
- Intravaginal manipulations by (German) osteopaths: a new low point for clinical research into alternative medicine?
- Visceral osteopathy is implausible and does not work … SO, LET’S FORGET ABOUT IT ONCE AND FOR ALL
Rigorous trials fail to show that it works for anything. So, the obvious solution to this dilemma is to conduct dodgy trials!
This study tested the effects of VM on dysmenorrhea, irregular, delayed, and/or absent menses, and premenstrual symptoms in PCOS patients.
Thirty Egyptian women with polycystic ovary syndrome (PCOS), with menstruation-related complaints and free from systematic diseases and/or adrenal gland abnormalities, participated in a single-blinded, randomized controlled trial. They were recruited from the women’s health outpatient clinic in the faculty of physical therapy at Cairo University, with an age of 20-34 years, and a body mass index (BMI) ≥25, <30 kg/m2. Patients were randomly allocated into two equal groups (15 patients); the control group received a low-calorie diet for 3 months, and the study group that received the same hypocaloric diet added to VM to the pelvic organs and their related structures for eight sessions over 3 months. Evaluations for body weight, BMI, and menstrual problems were done by weight-height scale, and menstruation-domain of Polycystic Ovary Syndrome Health-Related Quality of Life Questionnaire (PCOSQ), respectively, at baseline and after 3 months from interventions. Data were described as mean, standard deviation, range, and percentage whenever applicable.
Of 60 Egyptian women with PCOS, 30 patients were included, with baseline mean age, weight, BMI, and a menstruation domain score of 27.5 ± 2.2 years, 77.7 ± 4.3 kg, 28.6 ± 0.7 kg/m2, and 3.4 ± 1.0, respectively, for the control group, and 26.2 ± 4.7 years, 74.6 ± 3.5 kg, 28.2 ± 1.1 kg/m2, and 2.9 ± 1.0, respectively, for the study group. Out of the 15 patients in the study group, uterine adhesions were found in 14 patients (93.3%), followed by restricted uterine mobility in 13 patients (86.7%), restricted ovarian/broad ligament mobility (9, 60%), and restricted motility (6, 40%). At baseline, there was no significant difference (p>0.05) in any of the demographics (age, height), or dependent variables (weight, BMI, menstruation domain score) among both groups. Post-study, there was a statistically significant reduction (p=0.000) in weight, and BMI mean values for the diet group (71.2 ± 4.2 kg, and 26.4 ± 0.8 kg/m2, respectively) and the diet + VM group (69.2 ± 3.7 kg; 26.1 ± 0.9 kg/m2, respectively). For the improvement in the menstrual complaints, a significant increase (p<0.05) in the menstruation domain mean score was shown in the diet group (3.9 ± 1.0), and the diet + VM group (4.6 ± 0.5). On comparing both groups post-study, there was a statistically significant improvement (p=0.024) in the severity of menstruation-related problems in favor of the diet + VM group.
The authors concluded that VM yielded greater improvement in menstrual pain, irregularities, and premenstrual symptoms in PCOS patients when added to caloric restriction than utilizing the low-calorie diet alone in treating that condition.
WHERE TO START?
- Tiny sample size.
- A trail design (A+B vs B) which will inevitably generate a positive result.
- Questionable ethics.
VM is a relatively invasive and potentially embarrassing intervention for any woman; I imagine that this is all the more true in Egypt. In such circumstances, it is mandatory to ask whether a planned study is ethically justifiable. I would answer this question related to an implausible treatment like VM with a straight NO!
I realize that there may be people who disagree with me. But even those guys should accept that, at the very minimum, such a study must be designed such that it leads to a clear answer – is VM effective or not? The present trial merely suggests that the placebo effect associated with VM is powerful (which is hardly surprising for a therapy like VM).