herbal medicine
Few of us are aware of the fact that there are such things as alternative diagnoses, i.e. diagnoses used by practitioners of so-called alternative medicine (SCAM) that have no basis in science. They are nonetheless popular with some SCAM practitioners and usually cause a wide range of non-specific symptoms.
In part 1 of this series of posts, I dealt with:
- adrenal fatigue,
- candidiasis hypersensitivity,
- chronic intoxications.
Today I will briefly discuss three further alternative diagnoses.
Chronic Lyme Disease
Lyme disease exists, of course; it is a bacterial infection attained via the bite of a tick. By contrast chronic lyme disease is pure fantasy. It is often used to explain persistent pain, fatigue, and neurocognitive symptoms in patients without any evidence of previous acute lyme disease.
Once this diagnosis is given, prolonged treatment with multiple antimicrobial agents as well as a multitude of SCAMs are advocated. The range includes intravenous infusions of hydrogen peroxide, electromagnetic frequency treatments, garlic supplements, even stem cell transplants.
Unsurprisingly, none of them has been shown to work for chronic lyme disease.
Electromagnetic hypersensitivity
Electromagnetic hypersensitivity (EHS) is a condition where individuals report symptoms attributed to exposure to electromagnetic fields. It is not a recognized medical diagnosis.
Symptoms of EHS include headache, fatigue, stress, sleep disturbances, skin prickling, burning sensations and rashes, pain, psychological distress and many other health problems. The true case seems psychosomatic and unrelated to electromagnetic fields.
Practitioners nevertheless recommend all sorts of SCAMs including chelation, detox, diets, tocopherols , carotenoids, vitamin C, curcumin, resveratrol, flavonoids, sauna, blue light therapy none of which have been shown to be effective.
Homosexuality
Yes, it’s true: some SCAM practitioners offer treatments for homosexuality which must mean that they consider it to be a disease.
As reported in a previous blog post, the German ‘Association of Catholic Doctors’, Bund Katholischer Ärzte, claims that homeopathic remedies can cure homosexuality. On their website, they advise that ‘…the working group HOMEOPATHY of the Association notes homeopathic therapy options for homosexual tendencies…repertories contain special rubrics pointing to characteristic signs of homosexual behaviour, including sexual peculiarities such as anal intercourse.
Say no more!
It has been reported that 5 people who took a Japanese health supplement have died and more than 100 have been hospitalized as of Friday, a week after a pharmaceutical company issued a recall of the products, officials said. Osaka-based Kobayashi Pharmaceutical Co. came under fire for not going public quickly with problems known internally as early as January. Yet the first public announcement came only on 22 March. Company officials said 114 people were being treated in hospitals after taking products — including Benikoji Choleste Help meant to lower cholesterol — that contain an ingredient called benikoji, a red species of mold. Some people developed kidney problems after taking the supplements, but the exact cause was still under investigation in cooperation with government laboratories, according to the manufacturer.
“We apologize deeply,” President Akihiro Kobayashi told reporters last Friday, bowing for a long time to emphasize the apology alongside three other top company officials. He expressed remorse to those who have died and have been taken ill and to their families. He also apologized for the troubles caused to the entire health food industry and the medical profession, adding that the company was working to prevent further damage and improve crisis management.
The company’s products have been recalled — as have dozens of other products that contain benikoji, including miso paste, crackers, and a vinegar dressing. Japan’s health ministry put up a list on its official site of all the recalled products, including some that use benikoji for food coloring. The ministry warned the deaths could keep growing. The supplements could be bought at drug stores without a prescription from a doctor, and some may have been purchased or exported before the recall, including by tourists who may not be aware of the health risks.
Kobayashi Pharmaceutical had been selling benikoji products for years, with a million packages sold over the past 3 fiscal years, but a problem crept up with the supplements produced in 2023. Kobayashi Pharmaceutical said it produced 18.5 tons of benikoji last year. Some analysts blame the recent deregulation initiatives, which simplified and sped up approval for health products to spur economic growth.
________________________
Anouther source reported that Japanese authorities on Saturday raided a drug factory after a pharmaceutical company reported at least five deaths and 114 hospitalizations possibly linked to a health supplement. About a dozen Japanese health officials walked into the Osaka plant of the Kobayashi Pharmaceutical Co., as seen in footage of the raid widely telecasted on Japanese news. The health supplement in question is a pink pill called Benikoji Choleste Help. It is said to help lower cholesterol levels. A key ingredient is benikoji, a type of red mold. The company has said it knows little about the cause of the sickness, which can include kidney failure. It is currently investigating the effects in cooperation with Japan’s government.
___________________________
More recent reports update the figure of affected individuals: Japanese dietary supplements at the center of an expanding health scare have now been linked to at least 157 hospitalizations, a health ministry official said Tuesday.The figure reflects an increase from the 114 hospitalization cases that Kobayashi Pharmaceutical said on Friday were linked to its products containing red yeast rice, or beni kōji.
A Kobayashi Pharmaceutical spokeswoman confirmed the latest hospitalization cases without elaborating further.
Benikoji is widely sold and used; not just in Japan. It comes under a range of different names:
- red yeast rice,
- red fermented rice,
- red kojic rice,
- red koji rice,
- anka,
- angkak,
- Ben Cao Gang Mu.
It is a bright reddish purple fermented rice which acquires its color from being cultivated with the mold Monascus purpureus. Red yeast rice is used as food and as a medicine in Asian cultures, such as Kampo and TCM.
It contains lovastatin which, of course, became patented and is marketed as the prescription drug, Mevacor. Red yeast rice went on to become a non-prescription dietary supplement in the United States and other countries. In 1998, the U.S. FDA banned a dietary supplement containing red yeast rice extract, stating that red yeast rice products containing monacolin K are identical to a prescription drug, and thus subject to regulation as a drug.
The Amercian Medical Association (AMA) recently published a lengthy article on naturopathy in the US. Here are some excerpts:
There are three types of health professionals who offer naturopathic treatment:
- Naturopathic doctors. These nonphysicians graduate from a four-year, professional-level program at an accredited naturopathic medical school, earning either the doctor of naturopathy (ND) degree or the doctor of naturopathic medicine (NMD) degree.
- Traditional naturopaths, who have obtained education through some combination of a mentorship program with another professional or at an alternative clinic, distance-learning program or classroom schooling on natural health, or other holistic studies.
- Other health professionals such as chiropractors, massage therapists, dentists, nurses, nutritionists, or physicians who practice under a professional license but include some naturopathic methods in their practice and who may have studied on their own or taken courses on naturopathic methods.
At least 24 states and the District of Columbia regulate the practice of naturopathy. In order to be licensed, naturopaths in these states must earn an ND or NMD from an accredited naturopathic program and pass the Naturopathic Physicians Licensing Exam. Three states—Florida, South Carolina and Tennessee—prohibit the practice of naturopathy. In states that neither license nor prohibit the practice of naturopathy, traditional naturopaths and NDs alike may practice without being subject to state regulation.
Postgraduate training is neither common nor required of graduates of naturopathic schools, except in Utah … less than 10% of naturopaths participate in an approved residency, and such residencies last only a year and lack a high degree of standardization.
… naturopaths are required to get at least 1,200 hours of direct patient contact, physicians get 12,000–16,000 hours of clinical training…
ND programs emphasize naturopathic principes—for example, the healing power of nature—and naturopathic therapeutics such as botanical medicine, homeopoathy and hydrotherapy. Coursework in naturopathic therapeutics is combined with, and taught alongside, coursework in sciences. But there are no specifications around the number of hours required in each area … naturopathic students may lack exposure to key clinical scenarios in the course of their training … naturopathic students’ clinical experience is typically gained through outpatient health care clinics, as naturopathic medical schools typically do not have significant hospital affiliation. This means there is no guarantee that a naturopathic student completing a clinical rotation will see patients who are actually sick or hospitalized, and they may not be exposed to infants, children, adolescents or the elderly. It has been said that naturopaths tend to treat the “worried well.”
… Naturopaths claim they are trained as primary care providers and, as such, are educated and trained to diagnose, manage and treat many conditions, including bloodstream infections, heart disease and autoimmune disorders. Yet their education and training falls several years and thousands of hours short of what physicians get.
…The AMA believes it is the responsibility of policymakers to ensure that naturopaths’ claims that they can treat a broad range of conditions are backed by facts—facts that include the specific education and training necessary to ensure patient safety.
________________
The AMA is clearly cautious here. A less polite statement might simply stress that naturopaths are taught a lot of nonsense which they later tend to administer to their unsuspecting patients. On this blog, we have repeatedly discussed the danger naturopaths present to public health in the US and elsewhere, e.g.:
- How reliable are the claims made by naturopathic influencers?
- Naturopath jailed for selling fraudulent vaccination documents
- Naturopath fined for misdiagnosing and treating a rectal tumor for hemorrhoids
- Naturopaths are ‘not bound by science,’ lawyer argues
- Vaccination rates of Canadian healthcare professionals: those of chiropractors and naturopaths are at the lowest
- Is veterinary naturopathy animal abuse?
- Naturopathic ‘cancer specialist’ using coffee enemas found guilty
- Patients consulting chiropractors, homeopaths, or naturopaths are less likely to agree to the flu jab
- A naturopath responsible for the death of two cancer patients was sentenced to two years
- A naturopath in court after two of his cancer patients died
- Many naturopaths, homeopaths, and chiropractors are a risk to public health
- Naturopath treats autism with fecal transplants
- A naturopath promoting fake news about COVID vaccinations
- Naturopathy (according to the WNF) = quackery steeped in obsolete fantasies
- Canadian naturopaths may no longer call themselves ‘medically trained’
- Naturopaths’ counselling against vaccinations could be criminally negligent
- Naturopathy for cancer … claims that have the potential to be lethal
- Severe liver injury due to naturopaths’ prescription of Epsom salt
- Naturopaths should not treat children
- Some naturopaths are clearly a danger to public health
- Death of a child through naturopathy
Claims that naturopaths are a viable alternative to evidence-based medicine are wrong, irresponsible and dangerous. Regulators must be reminded that they have the duty to protect the public from charlatans and should therefore ensure that no false therapeutic or diagnostic claims can be made by naturopaths.
Looking at some ancient papers of mine, I came across a short BMJ paper from 1994. Here is a passage from it:
… A standard letter (on departmental letterhead) was written (in German) to all 189 firms that we identified as marketing herbal drugs in Germany. It asked (among other questions) for reprints of articles reporting controlled clinical trials on the company’s product(s).
Only 19 replies had reached us six weeks later. Four of these included at least one reprint. Twelve respondents regretted not knowing of clinical trials on their drug(s). In three cases we had written to a wrong address (one
instance) or to a firm which did not market phytomedicines (two instances).
These data, though far from conclusive, do not give the impression that research is in proportion to either prevalence or financial tumover of herbal remedies…
I wonder what the results would be, if we repeated this little excercise today, 30 years afteer the original investigation. I fear that the findings would be much the same or perhaps even worse. I also suspect that they would be similar regardless of the country we chose. Those who sell herbal remedies have very little incentive to do expensive clinical trials to test whether the products they earn their money with actually work. They may be doing well without it and ask themselves, why spend money on research that might not show what we hope and could easily turn out to jeopardize our financial success?
But the problem is by no means confined to herbal manufacturers (who would arguably have an important share to initiate and sponsor research). Even though fundamental questions remain unanswered, research into herbal medicine is scarce across the board.
To see whether this statement is true, I did a very quick Medline search. It showed that, in 2023, just over 13 000 papers on herbal medicine emerged. Of those, just 460 were listed as clinical trials. The latter figure is almost certainly considerably smaller than the true amount because Medline is over-generous in classifying papers as clinical trials. I thus estimate that only around 200 clinical trials of herbal medicine are conducted each year. Considering that we are dealing with thousands of herbs and ten thousands of herbal products, this figure is an embarrassment for the sector – which, as we have seen just days ago, is doing extremely well in finacial terms.
Here is the abstract of a recent article that I find worrying:
In 2020, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) challenged the world with a global outbreak that led to millions of deaths worldwide. Coronavirus disease 2019 (COVID-19) is the symptomatic manifestation of this virus, which can range from flu-like symptoms to utter clinical complications and even death. Since there was no clear medicine that could tackle this infection or lower its complications with minimal adverse effects on the patients’ health, the world health organization (WHO) developed awareness programs to lower the infection rate and limit the fast spread of this virus. Although vaccines have been developed as preventative tools, people still prefer going back to traditional herbal medicine, which provides remarkable health benefits that can either prevent the viral infection or limit the progression of severe symptoms through different mechanistic pathways with relatively insignificant side effects. This comprehensive review provides scientific evidence elucidating the effect of 10 different plants against SARS-CoV-2, paving the way for further studies to reconsider plant-based extracts, rich in bioactive compounds, into more advanced clinical assessments in order to identify their impact on patients suffering from COVID-19.
The conclusions of this paper read as follows:
…since these 10 herbs hold distinct bioactive compounds with significant properties in vitro and with remarkable benefits to human health, it is possible to prevent SARS-CoV-2 infection and reduce its symptomatic manifestations by consuming any of these 10 plants according to the recommended dose. The diversity in bioactive molecules between the different plants exerts various effects through different mechanisms at once, which makes it more potent than conventional synthetic drugs. Nonetheless, more studies are needed to highlight the clinical efficacy of these extracts and spot their possible side effects on patients, especially those with comorbidities who take multiple conventional drugs.
I should point out that the authors fail to offer a single reliable trial that would prove or even imply that any of the 10 herbal remedies can effectively treat or prevent COVID infections (to the best of my knowledge, no such studies exist). Laguage like “it is possible to prevent SARS-CoV-2 infection and reduce its symptomatic manifestations” is therefore not just misleading but highly dangerous and deeply unethical. Sadly, such evidence-free claims abound in herbal medicine.
I think the journal editor, the peer-reviewer, the authors and their universities ( University of Tripoli in Lebanon, American University of the Middle East, Egaila in Kuwait, University of Balamand, Kalhat, Tripoli in Lebanon, Lebanese University, Tripoli in Lebanon, Aix-Marseille Université in France) should be ashamed to produce such dangerous rubbish.
Traditional herbal medicine (THM) is frequently used in pediatric populations. This is perticularly true in many low-income countries. Yet THM has been associated with a range of adverse events, including liver toxicity, renal failure, and allergic reactions. Despite these concerns, its impact on multi-organ dysfunction syndrome (MODS) risk has so far not been thoroughly investigated.
This study aimed to investigate the incidence and predictors of MODS in a pediatric intensive care unit (PICU) in Ethiopia, with a focus on the association between THM use and the risk of MODS. It was designed as a single-center prospective cohort study conducted at a PICU in the university of Gondar Comprehensive Specialized hospital, Northwest Ethiopia. The researchers enrolled eligible patients aged one month to 18 years admitted to the PICU during the study period. Data on demographic characteristics, medical history, clinical and laboratory data, and outcome measures using standard case record forms, physical examination, and patient document reviews. The predictors of MODS were assessed using Cox proportional hazards models, with a focus on the association between traditional herbal medicine use and the risk of MODS.
A total of 310 patients were included in the final analysis, with a median age of 48 months and a male-to-female ratio of 1.5:1. The proportion and incidence of MODS were 30.96% (95% CI:25.8, 36.6) and 7.71(95% CI: 6.10, 9.40) per 100-person-day observation respectively. Renal failure (17.74%), neurologic failure (15.16%), and heart failure (14.52%) were the leading organ failures identified. Nearly one-third of patients (32.9%) died in the PICU, of which 59.8% had MODS. The rate of mortality was higher in patients with MODS than in those without. The Cox proportional hazards model identified renal disease (AHR = 6.32 (95%CI: 3.17,12.61)), intake of traditional herbal medication (AHR = 2.45, 95% CI:1.29,4.65), modified Pediatric Index of Mortality 2 (mPIM 2) score (AHR = 1.54 (95% CI: 1.38,1.71), and critical illness diagnoses (AHR = 2.68 (95% CI: 1.77,4.07)) as predictors of MODS.
The authors concluded that the incidence of MODS was high. Renal disease, THM use, mPIM 2 scores, and critical illness diagnoses were independent predictors of MODS. A more than twofold increase in the risk of MODS was seen in patients who used TMH. Healthcare providers should be aware of risks associated with THM, and educate caregivers about the potential harms of these products. Future studies with larger sample sizes and more comprehensive outcome measures are needed.
I do fully agree with the authors about the high usage of herbal and other so-called alternative medicines by children. We have shown that, in the UK the average one-year prevalence rate was 34% and the average lifetime prevalence was 42%. We have furthermore shown that the evidence base for these treatments in children is weak, even more so than for general populations. Finally, we can confirm that adverse effects are far from rare and often serious.
It is therefore high time, I think, that national regulators do more to protect children from SCAM practitioners who are at best uncritical about their treatments and at worse outright dangerous.
An alarming story of research fraud in the area of so-called alternative medicine (SCAM) is unfolding: Bharat B. Aggarwal, the Indian-American biochemist who worked at MD Anderson Cancer Center, focused his research on curcumin, a compound found in turmeric, and authored more than 125 Medline-listed articles about it. They reported that curcumin had therapeutic potential for a variety of diseases, including various cancers, Alzheimer’s disease and, more recently, COVID-19.
The last of these papers, entitled “Curcumin, inflammation, and neurological disorders: How are they linked?”, was publiched only a few months ago. Here is its abstract:
Background: Despite the extensive research in recent years, the current treatment modalities for neurological disorders are suboptimal. Curcumin, a polyphenol found in Curcuma genus, has been shown to mitigate the pathophysiology and clinical sequalae involved in neuroinflammation and neurodegenerative diseases.
Methods: We searched PubMed database for relevant publications on curcumin and its uses in treating neurological diseases. We also reviewed relevant clinical trials which appeared on searching PubMed database using ‘Curcumin and clinical trials’.
Results: This review details the pleiotropic immunomodulatory functions and neuroprotective properties of curcumin, its derivatives and formulations in various preclinical and clinical investigations. The effects of curcumin on neurodegenerative diseases such as Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), brain tumors, epilepsy, Huntington’s disorder (HD), ischemia, Parkinson’s disease (PD), multiple sclerosis (MS), and traumatic brain injury (TBI) with a major focus on associated signalling pathways have been thoroughly discussed.
Conclusion: This review demonstrates curcumin can suppress spinal neuroinflammation by modulating diverse astroglia mediated cascades, ensuring the treatment of neurological disorders.
The Anderson Cancer Center initially appeared to approve of Aggarwal’s work. However, in 2012, following concerns about image manipulation raised by pseudonymous sleuth Juuichi Jigen, MD Anderson Cancer Center launched a research fraud probe against Aggarwal which eventually led to 30 of Aggarwal’s articles being retracted. Moreover, PubPeer commenters have noted irregularities in many publications beyond the 30 that have already been retracted. Aggarwal thus retired from M.D. Anderson in 2015.
Curcumin doesn’t work well as a therapeutic agent for any disease – see, for instance, the summary from Nelson et al. 2017:
“[No] form of curcumin, or its closely related analogues, appears to possess the properties required for a good drug candidate (chemical stability, high water solubility, potent and selective target activity, high bioavailability, broad tissue distribution, stable metabolism, and low toxicity). The in vitro interference properties of curcumin do, however, offer many traps that can trick unprepared researchers into misinterpreting the results of their investigations.”
Despite curcumin’s apparent lack of therapeutic promise, the volume of research produced on curcumin grows each year. More than 2,000 studies involving the compound are now published annually. Many of these studies bear signs of fraud and involvement of paper mills. As of 2020, the United States National Institutes of Health (NIH) has spent more than 150 million USD funding projects related to curcumin.
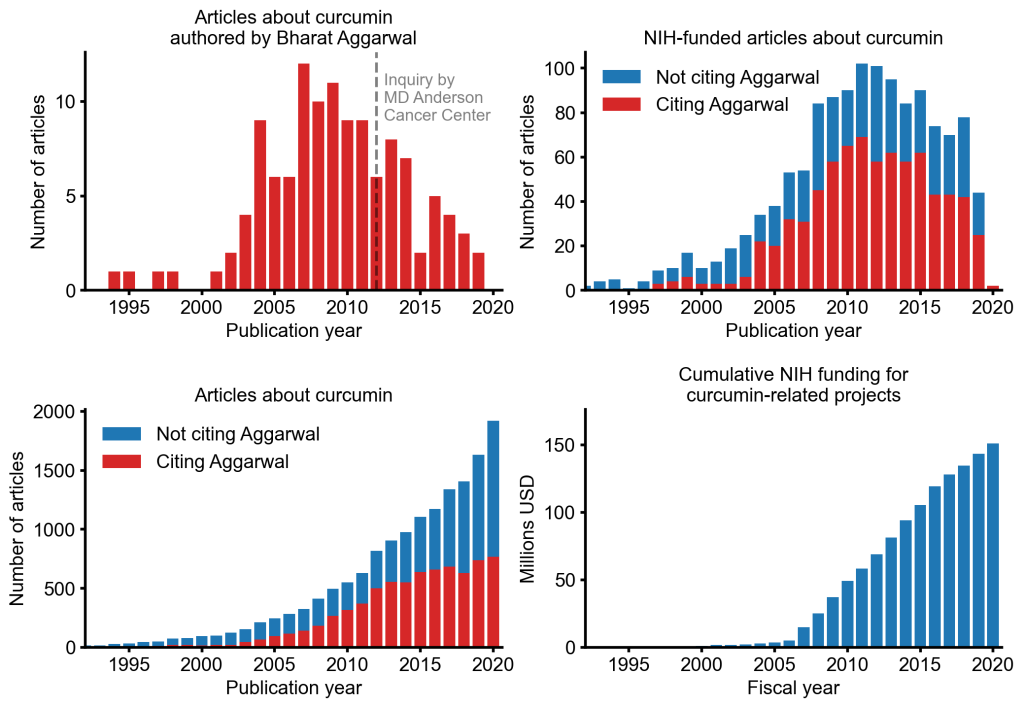
This proliferation of research has fueled curcumin’s popularity as a dietary supplement. It is estimated that the global market for curcumin as a supplement is around 30 million USD in 2020.
The damage done by this epic fraud is huge and far-reaching. Hundreds of millions of taxpayer dollars, countless hours spent toiling by junior scientists, thousands of laboratory animals sacrificed, thousands of cancer patients enrolled in clinical trials for ineffective treatments, and countless people who have eschewed effective cancer treatment in favor of curcumin, were encouraged by research steeped in lies.
In total, 404 respondents completed the survey, of which 254 (62.9%) treated cancer patients. Most practitioners were acupuncturists and herbalists (57.1%), had (16.8 ± 9.9) years of clinical experience and see a median of 2 (1, 4) cancer patients per week. Breast cancer (61.8%) is the most common cancer type seen in SCAM clinics. Adjunctive SCAM treatments are frequently concurrent with the patient’s cancer specific treatment (39.9%), which is also reflected by the main goal of a SCAM treatment to alleviate side effects (52.4%). However, only 28.0% of the respondents are in contact with the treating oncologist. According to the respondents, pain is most effectively treated using acupuncture, while herbal medicine is best for cancer-related fatigue. SCAM practitioners mostly use certified courses (33.1%) or online databases (28.3%) but often believe that experts are more reliable to inform their practice (37.0%) than research publications (32.7%).
The authors concluded that acupuncturists and herbalists commonly treat cancer patients. Most practitioners use SCAM as an adjunct to biomedicine as supportive care and use it largely in accordance with current oncological guidelines.
You would think that the combined expertise of these institutions are capable of producing a decent survey:
- Palliative Care Unit, Division of Oncology, Department of Internal Medicine, Medical University of Graz, 8036 Graz, Austria
- Northern College of Acupuncture, York YO1 6LJ, United Kingdom
- School of Health and Society, Faculty of Education, Health and Wellbeing, University of Wolverhampton, Wolverhampton WV1 1LY, United Kingdom
- National Institute of Complementary Medicine Health Research Institute, Western Sydney University, Penrith NSW 2751, Australia
- Translational Health Research Institute, Western Sydney University, Penrith NSW 2751, Australia
- Medical Research Institute of New Zealand, Wellington 6021, New Zealand
- Translational Oncology, University Hospital of Augsburg, 86156 Augsburg, Germany
Well, you would have been mistaken! This surely is one of the worst investigations I have seen for a while. Here are just three reasons why:
- The researchers designed an anonymous self-completion questionnaire collecting data about the participating practitioners’ demographics and clinical practice of integrative oncology. Someone should tell them that one ought to validate questionnairs before using them and that validated questionnairs exist. Unvalidated questionnairs cannot tell us much of value.
- The researchers invited SCAM practitioners in Austria, Germany, USA, Australia, and New Zealand to participate in this study. Invitations were distributed through social media and emails between October 2022 and December 2022 by professional organizations. Someone should tell them that research needs to be reproducible and surveys need to cover a representative population – both criteria that are not met here.
- The survey participants had to hold a valid license to perform acupuncture, herbal medicine, or both. That excludes all other SCAM practitioners.
Despite these serious flaws, the survey shows two findings that might be worth mentioning:
- only 28.0% of the SCAM practitioners were in contact with the treating oncologist;
- SCAM practitioners believe that “experts” are more reliable to inform their practice than research publications.
For me, these two points alone would be sufficient reason to run a mile!
The NZZ recently published a long and horrific report about a natural health clinic and its doctors. Here is a version translated and shortened by me; perhaps it makes a few people think twice before they waste their money and risk their health:
It is a narrow mountain road that they are racing down on this spring evening. Over the green Appenzell hills, towards Herisau hospital. Kathrin Pfister* is fighting for her life in the car. At the wheel is Thomas Rau, internationally renowned practitioner of so-called alternative medicine (SCAM) and director of his own luxury clinic, the Biomed Centre Sonnenberg. Three days later, Kathrin Pfister is dead. The most likely finding according to the experts: Pfister was injected with a drug that was not authorised in Switzerland at the time, the side effects of which killed her.
Pfister is not the only woman to have lost her life following treatment at the Sonnenberg. Other experts accuse Rau of serious breaches of duty that led to the death of a patient. Rau and another doctor are thus being investigated for involuntary manslaughter.
The events remained hidden from the public for over two years. It’s not just about one doctor, not just about one clinic. The events are politically explosive for Appenzell Ausserrhoden. The canton is the centre of alternative medicine in Switzerland. SCAM doctors are an important economic factor in Ausserrhoden. Wealthy people from all over the world fly here for therapies that most conventional doctors just shake their heads at. Treatments lasting several weeks with a hotel stay cost five-figure sums.
The 73-year-old Dr Rau is the star among Swiss alternative medicine practitioners.He describes himself as the “Mozart of medicine”. The Biomed Centre Sonnenberg is “Mozart’s” last big project. The clinic has a hotel and gluten-free vegan restaurant from the Tibits chain. Even the feather pillows are replaced with bamboo ones. All for the “detox” that Rau praises.
Kathrin Pfister’s case began in mid-April 2021, just four months after the Sonnenberg centre opened. She is actually healthy and comes to the clinic anyway; because of some digestive problems and headaches. The hospital records show that Pfister received infusions. Initially only those containing vitamin C and homeopathic remedies. Then one with artesunate, a preparation against malaria. And finally, on a Friday, Pfister was injected with a solution of alpha-lipoic acid into his bloodstream. The infusion is used in Germany for long-term diabetics with nerve damage. It was not authorised as a medicinal product in Switzerland at the time. According to the forensic experts, it was this substance that was “ultimately causally linked to the death”.
A few hours later, Pfister had severe abdominal cramps. Then pain throughout the body. The number of platelets in her blood drops dramatically. Anxiety sets in at the clinic. The intensive care doctors in Herisau and later at the cantonal hospital in St. Gallen can do nothing more. Pfister had a massive blood clotting disorder. Her liver and kidneys were no longer functioning.
Mary Anne Hawrylak meets Thomas Rau by chance at the clinic that weekend. She too is a patient, recently flown in from the USA. Hawrylak had massive side effects after infusions that Friday. “When I told him about it, he turned white as a sheet, like a ghost,” says Hawrylak. “Doctor Rau told me in horror that I had received the same infusions as ‘Kathrin’ and that he had to test my blood.” The tests showed that her blood platelet count had also dropped, says Hawrylak.
The forensic experts point to a central fact: Alpha lipoic acid can cause blood clotting disorders. They come to the conclusion that this is “most likely a lethal side effect of a drug”. The use of drugs that are not authorised in Switzerland is legal if they are authorised in a country with a comparable procedure. However, there is no real reason to inject this medication into the bloodstream of healthy people. It was authorised in Germany for diabetes patients with nerve damage. So, Pfister did not have this authorisation.
Experts refer to such applications as “off-label use”. Off-label treatments should only be carried out “on the basis of valid guidelines, generally recognised recommendations or scientific literature”. The guidelines also require that patients are given comprehensive information about off-label use. This counselling session should be documented in writing. None of this can be found in the clinic’s files. No written consent, no documented risk-benefit assessment, no reference to the risk of blood clotting disorders. The forensic experts state: “The scant documentation from the Sonnenberg Biomed Centre does not contain any corresponding information document.” The question arises as to “whether the medical treatment at the Sonnenberg Biomed Centre was carried out with the necessary medical care”.
Patient Hawrylak also says: “I was not told exactly what was in the infusions. I was never told that the medication was not authorised in Switzerland or that its use was off-label. I spoke to Dr Rau about what had happened to ‘Kathrin’ because I was worried about myself,” says Hawrylak. “He said to me: ‘I don’t think it was the infusions. I think it was the Covid vaccinations.” He only justified this with his “intuition”.
The Pfister case triggered an investigation by the public prosecutor’s office. But what hardly anyone knew at the time was that it was not the first questionable death at the clinic – not even the first in a month. Ruth Schmid*, a 77-year-old Swiss woman, had died just three weeks earlier. In this case, the forensic pathologists accused Rau: He had made mistakes that not even a medical student should have made, thus causing Schmid’s death.
Schmid was also in the clinic for a kind of cure. When she was about to leave, she began to tremble violently and had extreme stomach pains. She screamed “like an animal”, her partner said during the interrogation. Ultrasound examinations were carried out at the clinic and Rau gave Schmid painkillers, including morphine. According to the partner’s statement to the public prosecutor’s office, he asked Rau whether Schmid needed to be taken to hospital. Rau said no. Schmid stayed in the hotel room overnight. The next day – according to Rau, she had been feeling better since the previous evening – she travelled home. According to Rau’s confiscated notes, “she was to report closely” and return in four days. At home, Ruth Schmid fell into a coma-like state overnight. Admitted to Zurich University Hospital in an emergency, Schmid died there of cardiovascular failure due to septic shock.
The Zurich forensic pathologists performed an autopsy on Schmid’s body. Their findings: Schmid had suffered from intestinal paralysis. As a result, bacteria entered her body and poisoned her blood, leading to a heart attack. “From a forensic medical point of view, it is incomprehensible why the attending physician, Dr Thomas Rau, did not carry out appropriate diagnostics.” The irritation of the forensic experts is evident in almost every line. There had been several warning signs of intestinal paralysis. The forensic experts wrote: “This knowledge is taught in medical school and is considered basic knowledge in human medicine.” Rau’s behaviour was “a breach of the doctor’s duty of care”. With timely treatment, the prognosis for intestinal paralysis is excellent. The sad conclusion: Ruth Schmid did not have to die.
During questioning by the public prosecutor’s office, Rau denied any guilt. Schmid had left in “good condition”. There was no causality between what happened in the clinic and the death. The findings and conclusions of the Zurich forensic pathologists were wrong. Schmid did not have intestinal paralysis or septicaemia. He had been able to rule out intestinal paralysis because intestinal noises had been audible in the morning. The dose of morphine had been very small, so that it had had no effect. There were no indications of a serious condition. Rau testified that he had acted professionally, as would be expected of an internal medicine doctor.
In the Kathrin Pfister case, the doctors treating her also deny any culpability and question the forensic medical report. The doctor’s lawyer writes that the criminal investigation will show that there was no breach of the doctor’s duty to provide information. Alpha-lipoic acid was not responsible for the death. The expert opinion is not convincing in terms of method or content: “When analysed in depth, it contains no justification that the use of alpha-lipoic acid was in any way causal for the patient’s death.”
During the hearing on the Pfister case, Rau said that restricting the use of alpha-lipoic acid to diabetics was “a joke” and far too narrowly defined. He claimed that Pfister had polyneuropathy, a complex nerve disease. However, there is no mention of this in the files of Rau’s clinic.
The criminal investigation is ongoing in both cases. But did more happen on the Sonnenberg? A former hospital employee, who independently reported to the police, told the public prosecutor about other hair-raising incidents. During the interrogation, she testified that she had seen a young woman being carried out of the clinic extremely weak after an infusion. Days later, she had overheard parts of a telephone conversation between Rau and the patient’s angry husband which made it clear that the woman had died. The former employee also recounted a conversation with Rau’s wife, who is a trained nurse. She said that she had driven a patient to a hospital in Zurich in a private car with Rau because Rau was determined to take her to a particular specialist. The patient was so unwell that she was afraid the woman would die on the way. If this is true, Rau would have travelled past several hospitals with a seriously ill patient.
Hawrylak has one last memory of Appenzell etched in his memory. The departure. She was just leaving the clinic when Rau wished her good luck: “I could only say to him: I wish you good luck too, Doctor Rau. I think you’re really going to need it.”
*Names were altered.
Need to find a last-minute Christmas present?
What about the ‘libido-boosting soft drink G Spot? That surely would make a wonderful holiday season (provided the drink does not get highjacked by your mother-in-law). Here is more info, in case you are interested:
Launched in May 2023, the line of plant-based sparkling drinks include “life-enhancing adaptogens and nootropics that invigorate and boost performance and cognitive functions”.
“I’ve had a serious soft drink habit for the past 20 years, and I don’t drink enough water,” Gillian Anderson (OBE), the firm’s founder and brain behind the drinks, said. “I know sugar and caffeine are not good for me, but I haven’t found an alternative that has the same effect. And although I love the idea of flavoured water, I really don’t like the taste of what’s out there. So, I thought, if what I’m looking for doesn’t exist, why don’t I make it?”
Arouse, specifically, has been designed to “awaken your senses” with Passionfruit, White Peach and Habanero, blended expertly with functional ingredients, including: Butterfly Pea (Clitoria ternatea), a plant species used in traditional medicine as an aphrodisiac; I-Arginine and I-Citrulline, amino acids which increase blood flow to sexual organs to improve libido; and Vitamin B6, a nutrient that regulates sexual hormones.
“At G Spot, we believe wellness is due a fresh take. One that’s less intense and anxiety inducing. One that truly makes you feel good, without guilt or inhibition. Our newest drink, Arouse, embodies our commitment to this philosophy. Arouse isn’t just a flavour; it’s an experience!” says CEO Rebekah Hall.
Named after their intended effects, the rest of the line comes in the form of Lift, combining berry, apply and peppercorn flavors with the wellbeing benefits of bacopa, theanine, cordyceps, and lion’s mane (boosting energy levels, stamina, and brain power while also combating stress); Protect, blending meadowsweet, ginger, lemon, turmeric, peppercorn and chaga (offering additional immune system-boosting benefits)’ and Soothe, which combines apples, sage, and cornflower with magnesium, maca, reishi, and ashwagandha (which works to relax the body as it benefits from the brand-wide libido boost).
All G Spot drinks available online in 6- and 12-pack bundles (250ml per can), as well as in Harvey Nichols (in-store and online) in the UK.
Prices range from £3.50 to £29.99 ($4.28 to $36.54).
The drinks are advertised to invigorate and boost performance and cognitive functions.
Any evidence for these medicinal claims?
Sadly not – at least I could not find any.
A case for the Advertising Standards Agency (ASA)?
Possibly!

