methodology
Guided imagery is said to distract patients from disturbing feelings and thoughts, positively affects emotional well-being, and reduce pain by producing pleasing mental images.
This study aimed to determine the effects of guided imagery on postoperative pain management in patients undergoing lower extremity surgery. This randomized controlled study was conducted between April 2018 and May 2019. It included 60 patients who underwent lower extremity surgery. After using guided imagery, the posttest mean Visual Analog Scale score of patients in the intervention group was found to be 2.56 (1.00 ± 6.00), whereas the posttest mean score of patients in the control group was 4.10 (3.00 ± 6.00), and the difference between the groups was statistically significant (p <.001).
The authors concluded that guided imagery reduces short-term postoperative pain after lower extremity surgery.
I did not want to spend $52 to access the full article. Therefore, I can only comment on what the abstract tells me – and that is regrettably not a lot.
In fact, we don’t even learn what treatment was given to the control group. I guess that both groups receive standard post-op care and the control group received nothing in addition. This would mean that the observed effect might be entirely due to placebo and other non-specific effects. If that is so, the authors’ conclusion is not accurate.
I happen to think that guided imagery is a promising albeit under-researched therapy. Therefore, I am particularly frustrated to see that the few trials that do emerge of this option are woefully inadequate to determine its value.
Sure, the LP is dangerous nonsense, but this begs the question of whether so-called alternative medicine (SCAM) has anything to offer for patients suffering from ME/CFS. If the LP story tells us anything, then it must be this: we should not trust single trials, particularly if they seem dodgy. In other words, we should look at systematic reviews that synthesize ALL clinical trials and evaluate them critically.
To locate this type of evidence I conducted several Medline searches and found several recent systematic reviews that address the issue:
Context: A variety of interventions have been used in the treatment and management of chronic fatigue syndrome (CFS). Currently, debate exists among health care professionals and patients about appropriate strategies for management.
Objective: To assess the effectiveness of all interventions that have been evaluated for use in the treatment or management of CFS in adults or children.
Data sources: Nineteen specialist databases were searched from inception to either January or July 2000 for published or unpublished studies in any language. The search was updated through October 2000 using PubMed. Other sources included scanning citations, Internet searching, contacting experts, and online requests for articles.
Study selection: Controlled trials (randomized or nonrandomized) that evaluated interventions in patients diagnosed as having CFS according to any criteria were included. Study inclusion was assessed independently by 2 reviewers. Of 350 studies initially identified, 44 met inclusion criteria, including 36 randomized controlled trials and 8 controlled trials.
Data extraction: Data extraction was conducted by 1 reviewer and checked by a second. Validity assessment was carried out by 2 reviewers with disagreements resolved by consensus. A qualitative synthesis was carried out and studies were grouped according to type of intervention and outcomes assessed.
Data synthesis: The number of participants included in each trial ranged from 12 to 326, with a total of 2801 participants included in the 44 trials combined. Across the studies, 38 different outcomes were evaluated using about 130 different scales or types of measurement. Studies were grouped into 6 different categories. In the behavioral category, graded exercise therapy and cognitive behavioral therapy showed positive results and also scored highly on the validity assessment. In the immunological category, both immunoglobulin and hydrocortisone showed some limited effects but, overall, the evidence was inconclusive. There was insufficient evidence about effectiveness in the other 4 categories (pharmacological, supplements, complementary/alternative, and other interventions).
Conclusions: Overall, the interventions demonstrated mixed results in terms of effectiveness. All conclusions about effectiveness should be considered together with the methodological inadequacies of the studies. Interventions which have shown promising results include cognitive behavioral therapy and graded exercise therapy. Further research into these and other treatments is required using standardized outcome measures.
Introduction: Chronic fatigue syndrome (CFS) affects between 0.006% and 3% of the population depending on the criteria of definition used, with women being at higher risk than men.
Methods and outcomes: We conducted a systematic review and aimed to answer the following clinical question: What are the effects of treatments for chronic fatigue syndrome? We searched: Medline, Embase, The Cochrane Library, and other important databases up to March 2010 (Clinical Evidence reviews are updated periodically; please check our website for the most up-to-date version of this review). We included harms alerts from relevant organisations such as the US Food and Drug Administration (FDA) and the UK Medicines and Healthcare products Regulatory Agency (MHRA).
Results: We found 46 systematic reviews, RCTs, or observational studies that met our inclusion criteria. We performed a GRADE evaluation of the quality of evidence for interventions.
Conclusions: In this systematic review we present information relating to the effectiveness and safety of the following interventions: antidepressants, cognitive behavioural therapy (CBT), corticosteroids, dietary supplements, evening primrose oil, galantamine, graded exercise therapy, homeopathy, immunotherapy, intramuscular magnesium, oral nicotinamide adenine dinucleotide, and prolonged rest.
Background: Throughout the world, patients with chronic diseases/illnesses use complementary and alternative medicines (CAM). The use of CAM is also substantial among patients with diseases/illnesses of unknown aetiology. Chronic fatigue syndrome (CFS), also termed myalgic encephalomyelitis (ME), is no exception. Hence, a systematic review of randomised controlled trials of CAM treatments in patients with CFS/ME was undertaken to summarise the existing evidence from RCTs of CAM treatments in this patient population.
Methods: Seventeen data sources were searched up to 13th August 2011. All randomised controlled trials (RCTs) of any type of CAM therapy used for treating CFS were included, with the exception of acupuncture and complex herbal medicines; studies were included regardless of blinding. Controlled clinical trials, uncontrolled observational studies, and case studies were excluded.
Results: A total of 26 RCTs, which included 3,273 participants, met our inclusion criteria. The CAM therapy from the RCTs included the following: mind-body medicine, distant healing, massage, tuina and tai chi, homeopathy, ginseng, and dietary supplementation. Studies of qigong, massage and tuina were demonstrated to have positive effects, whereas distant healing failed to do so. Compared with placebo, homeopathy also had insufficient evidence of symptom improvement in CFS. Seventeen studies tested supplements for CFS. Most of the supplements failed to show beneficial effects for CFS, with the exception of NADH and magnesium.
Conclusions: The results of our systematic review provide limited evidence for the effectiveness of CAM therapy in relieving symptoms of CFS. However, we are not able to draw firm conclusions concerning CAM therapy for CFS due to the limited number of RCTs for each therapy, the small sample size of each study and the high risk of bias in these trials. Further rigorous RCTs that focus on promising CAM therapies are warranted.
Background: There is no curative treatment for chronic fatigue syndrome (CFS). Traditional Chinese medicine (TCM) is widely used in the treatment of CFS in China.
Objective: To evaluate the effectiveness and safety of TCM for CFS.
Methods: The protocol of this review is registered at PROSPERO. We searched six main databases for randomized clinical trials (RCTs) on TCM for CFS from their inception to September 2013. The Cochrane risk of bias tool was used to assess the methodological quality. We used RevMan 5.1 to synthesize the results.
Results: 23 RCTs involving 1776 participants were identified. The risk of bias of the included studies was high. The types of TCM interventions varied, including Chinese herbal medicine, acupuncture, qigong, moxibustion, and acupoint application. The results of meta-analyses and several individual studies showed that TCM alone or in combination with other interventions significantly alleviated fatigue symptoms as measured by Chalder’s fatigue scale, fatigue severity scale, fatigue assessment instrument by Joseph E. Schwartz, Bell’s fatigue scale, and guiding principle of clinical research on new drugs of TCM for fatigue symptom. There was no enough evidence that TCM could improve the quality of life for CFS patients. The included studies did not report serious adverse events.
Conclusions: TCM appears to be effective to alleviate the fatigue symptom for people with CFS. However, due to the high risk of bias of the included studies, larger, well-designed studies are needed to confirm the potential benefit in the future.
Background: As the etiology of chronic fatigue syndrome (CFS) is unclear and the treatment is still a big issue. There exists a wide range of literature about acupuncture and moxibustion (AM) for CFS in traditional Chinese medicine (TCM). But there are certain doubts as well in the effectiveness of its treatment due to the lack of a comprehensive and evidence-based medical proof to dispel the misgivings. Current study evaluated systematically the effectiveness of acupuncture and moxibustion treatments on CFS, and clarified the difference among them and Chinese herbal medicine, western medicine and sham-acupuncture.
Methods: We comprehensively reviewed literature including PubMed, EMBASE, Cochrane library, CBM (Chinese Biomedical Literature Database) and CNKI (China National Knowledge Infrastructure) up to May 2016, for RCT clinical research on CFS treated by acupuncture and moxibustion. Traditional direct meta-analysis was adopted to analyze the difference between AM and other treatments. Analysis was performed based on the treatment in experiment and control groups. Network meta-analysis was adopted to make comprehensive comparisons between any two kinds of treatments. The primary outcome was total effective rate, while relative risks (RR) and 95% confidence intervals (CI) were used as the final pooled statistics.
Results: A total of 31 randomized controlled trials (RCTs) were enrolled in analyses. In traditional direct meta-analysis, we found that in comparison to Chinese herbal medicine, CbAM (combined acupuncture and moxibustion, which meant two or more types of acupuncture and moxibustion were adopted) had a higher total effective rate (RR (95% CI), 1.17 (1.09 ~ 1.25)). Compared with Chinese herbal medicine, western medicine and sham-acupuncture, SAM (single acupuncture or single moxibustion) had a higher total effective rate, with RR (95% CI) of 1.22 (1.14 ~ 1.30), 1.51 (1.31-1.74), 5.90 (3.64-9.56). In addition, compared with SAM, CbAM had a higher total effective rate (RR (95% CI), 1.23 (1.12 ~ 1.36)). In network meta-analyses, similar results were recorded. Subsequently, we ranked all treatments from high to low effective rate and the order was CbAM, SAM, Chinese herbal medicine, western medicine and sham-acupuncture.
Conclusions: In the treatment of CFS, CbAM and SAM may have better effect than other treatments. However, the included trials have relatively poor quality, hence high quality studies are needed to confirm our finding.
Objectives: This meta-analysis aimed to assess the effectiveness and safety of Chinese herbal medicine (CHM) in treating chronic fatigue syndrome (CFS). Methods: Nine electronic databases were searched from inception to May 2022. Two reviewers screened studies, extracted the data, and assessed the risk of bias independently. The meta-analysis was performed using the Stata 12.0 software. Results: Eighty-four RCTs that explored the efficacy of 69 kinds of Chinese herbal formulas with various dosage forms (decoction, granule, oral liquid, pill, ointment, capsule, and herbal porridge), involving 6,944 participants were identified. This meta-analysis showed that the application of CHM for CFS can decrease Fatigue Scale scores (WMD: -1.77; 95%CI: -1.96 to -1.57; p < 0.001), Fatigue Assessment Instrument scores (WMD: -15.75; 95%CI: -26.89 to -4.61; p < 0.01), Self-Rating Scale of mental state scores (WMD: -9.72; 95%CI:-12.26 to -7.18; p < 0.001), Self-Rating Anxiety Scale scores (WMD: -7.07; 95%CI: -9.96 to -4.19; p < 0.001), Self-Rating Depression Scale scores (WMD: -5.45; 95%CI: -6.82 to -4.08; p < 0.001), and clinical symptom scores (WMD: -5.37; 95%CI: -6.13 to -4.60; p < 0.001) and improve IGA (WMD: 0.30; 95%CI: 0.20-0.41; p < 0.001), IGG (WMD: 1.74; 95%CI: 0.87-2.62; p < 0.001), IGM (WMD: 0.21; 95%CI: 0.14-0.29; p < 0.001), and the effective rate (RR = 1.41; 95%CI: 1.33-1.49; p < 0.001). However, natural killer cell levels did not change significantly. The included studies did not report any serious adverse events. In addition, the methodology quality of the included RCTs was generally not high. Conclusion: Our study showed that CHM seems to be effective and safe in the treatment of CFS. However, given the poor quality of reports from these studies, the results should be interpreted cautiously. More international multi-centered, double-blinded, well-designed, randomized controlled trials are needed in future research.
What does all that tell us?
Disappointingly, it tells me that SCAM has preciously little to offer for ME/CFS patients.
But what about the TCM treatments? Aren’t the above reviews quite positive TCM?
Yes, they are but I nevertheless recommend taking them with a healthy pinch of salt.
Why?
Because we have seen many times before that, for a range of reasons, Chinese researchers of TCM draw false positive conclusions. That may sound unfair, harsh, or even racist, but I think it’s true. If you disagree, please show me a couple of systematic reviews of TCM for any human disease by Chinese researchers that have drawn negative conclusions.
And what is my advice to patients suffering from ME/CSF?
I think the best I can offer is this: be very cautious about the many claims made by SCAM enthusiasts; if it sounds too good to be true, it probably is!
The Lightning Process (LP) is a therapy for ME based on ideas from osteopathy, life coaching, and neuro-linguistic programming. LP is claimed to work by teaching people to use their brains to “stimulate health-promoting neural pathways”. One young patient once described it as follows: “Whenever you get a negative thought, emotional symptom, you are supposed to turn on one side and with your arm movements in a kind if stop motion, just say STOP very firmly and that is supposed to cut off the adrenaline response.”
Allegedly, the LP teaches individuals to recognize when they are stimulating or triggering unhelpful physiological responses and to avoid these, using a set of standardized questions, new language patterns, and physical movements with the aim of improving a more appropriate response to situations. The LP involves three group sessions on consecutive days where participants are taught theories and skills, which are then practiced through simple steps, posture, and coaching.
Does LP work?
Some think it does, particularly in Norway, it seems.
Proponents of the ‘LP’ in Norway claim that 90% of all ME patients get better after trying it. However, such claims seem to be more than questionable.
- In the Norwegian ME association’s user survey from 2012 with 1,096 participants, 164 ME patients stated that they had tried LP. 21% of these patients experienced improvement or great improvement and 48% got worse or much worse.
- In Norway’s National Research Center in Complementary and Alternative Medicine, NAFKAM’s survey from 2015 amongst 76 patients 8 had a positive effect and 5 got worse or much worse.
- A survey by the Norwegian research foundation, published in the journal Psykologisk, with 660 participants, showed that 62 patients had tried LP, and 5 were very or fairly satisfied with the results.
Such figures seem to reflect the natural history of the condition and may be totally unrelated to LP.
The LP instructors’ claims of a 90% positive effect are used for marketing and for lobbying. Their aim is to influence politicians, health authorities, and welfare and disability benefits authorities. They want to get the LP course approved as part of the public health service.
The company ‘Aktiv Prosess’ was started by LP instructors Live Landmark and Vibeke C. Hammer. In an article in the Norwegian medical journal Tidsskriftet in 2016, Landmark describes her own customer satisfaction survey from 2008 as «generating a hypothesis». Landmark has also written a book about her personal story and holds lectures for medical students, medical doctors, and nurses. Now she is trying to run a clinical trial which, many experts believe, is far from rigorous and set up to produce a positive result.
Positive experiences with LP have received massive media coverage for 15 years. Anecdotes are recycled in the media and give the impression of being a higher number than reality. We rarely hear about those who deteriorated: https://lp-fortellinger.no/ (English language link here).
The NICE guidelines for ME/CFS specifically (and in my view rightly) warn against offering LP to ME patients.
Menopausal symptoms are a domaine of so-called alternative medicine (SCAM), not least because many women are worried about hormone treatments and therefore want ‘something natural’. TCM practitioners are only too keen to offer their services. But do their treatments really work?
This study aimed to analyze the effectiveness of acupuncture combined with Chinese herbal medicine (CHM) on mood disorder symptoms for menopausal women.
A total of 95 qualified Chinese participants were randomly assigned to one of three groups:
- 31 in the acupuncture combined with CHM group (combined group),
- 32 in the acupuncture combined with CHM placebo group (acupuncture group),
- 32 in the CHM combined with sham acupuncture group (CHM group).
The patients were treated for 8 weeks and followed up for 4 weeks. The data were collected using the Greene Climacteric Scale (GCS), self-rating depression scale (SDS), self-rating anxiety scale (SAS), and safety index.
The three groups each showed significant decreases in the GCS, SDS, and SAS after treatment (p < 0.05). Furthermore, the effect on the GCS total score and the anxiety domain lasted until the follow-up period in the combined group (p < 0.05). Within the three groups, there was no difference in GCS and SAS between the three groups after treatment (p > 0.05). However, the combined group showed significant improvement in the SDS, compared with both the acupuncture group and the CHM group at 8 weeks and 12 weeks (p < 0.05). No obvious abnormal cases were found in any of the safety indexes.
The authors concluded that the results suggest that either acupuncture, or CHM or combined therapy offer safe improvement of mood disorder symptoms for menopausal women. However, the combination therapy was associated with more stable effects in the follow-up period and a superior effect on improving depression symptoms.
Previous reviews have drawn conclusions that are far less positive, e.g.:
- the observed clinical benefit associated with acupuncture may be due, in part, or in whole to nonspecific effects.
- the evidence gathered was not sufficient to affirm the effectiveness of traditional acupuncture compared with sham acupuncture.
- For natural menopause, one large study has shown acupuncture to be superior to self-care alone in reducing the number of hot flushes and improving the quality of life; five small studies have been unable to demonstrate that the effect of acupuncture is limited to any particular points, as traditional theory would suggest; and one study showed acupuncture was superior to blunt needle for flash frequency but not intensity.
- Sham-controlled RCTs fail to show specific effects of acupuncture for control of menopausal hot flushes.
It seems therefore wise to take the conclusions of the new study with a pinch of salt. The intergroup difference observed in this trial may well be due to residual biases, multiple testing, or coincidence. And the reported intragroup differences are in complete accord with the fact that the employed therapies are mere placebos.
This, of course, begs the question of whether SCAM has anything else to offer for women suffering from menopausal symptoms. To answer it, I can refer you to one of our systematic reviews:
Some evidence exists in favour of phytosterols and phytostanols for diminishing LDL and total cholesterol in postmenopausal women. Similarly, regular fiber intake is effective in reducing serum total cholesterol in hypercholesterolemic postmenopausal women. Clinical evidence also exists on the effectiveness of vitamin K, a combination of calcium and vitamin D or a combination of walking with other weight-bearing exercise in reducing bone mineral density loss and the incidence of fractures in postmenopausal women. Black cohosh appears to be effective therapy for relieving menopausal symptoms, primarily hot flashes, in early menopause. Phytoestrogen extracts, including isoflavones and lignans, appear to have only minimal effect on hot flashes but have other positive health effects, e.g. on plasma lipid levels and bone loss. For other commonly used CAMs, e.g. probiotics, prebiotics, acupuncture, homeopathy and DHEA-S, randomized, placebo-controlled trials are scarce and the evidence is unconvincing. More and better RCTs testing the effectiveness of these treatments are needed.
Hemiparesis is a severe impairment following a stroke that affects the majority of stroke patients. Rehabilitation is usually at least partly successful. But might results be improved with homeopathy?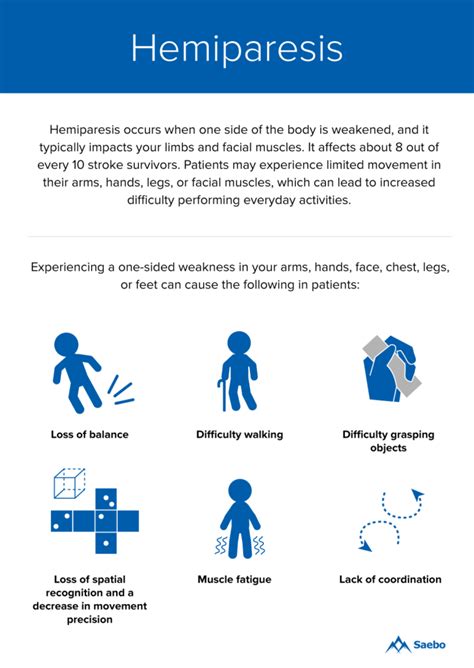
This trial tested the efficacy of individualized homeopathic medicines (IHMs) in comparison with identical-looking placebos in the treatment of post-stroke hemiparesis (PSH) in the mutual context of standard physiotherapy (SP).
A 3-months, open-label, randomized, placebo-controlled trial (n = 60) was conducted at the Organon of Medicine outpatient departments of the ‘National Institute of Homoeopathy’, West Bengal, India. Patients were randomized to receive IHMs plus SP (n = 30) or identical-looking placebos plus SP (n = 30). The primary outcome measure was Medical Research Council (MRC) muscle strength grading scale; secondary outcomes were Stroke Impact Scale (SIS) version 2.0, Modified Ashworth Scale (MAS), and stroke recovery 0-100 visual analog scale (VAS) scores; all measured at baseline and 3 months after the intervention. Group differences and effect sizes (Cohen’s d) were calculated on the intention-to-treat sample.
Although overall improvements were higher in the IHMs group than in the placebo group with small to medium effect sizes, the group differences were statistically non-significant (all P>0.05, unpaired t-tests). Improvement in SIS physical problems was significantly higher with IHM than with placebo (mean difference 2.0, 95% confidence interval 0.3 to 3.8, P = 0.025, unpaired t-test). Causticum, Lachesis mutus, and Nux vomica were the most frequently prescribed medicines. No harms, unintended effects, homeopathic aggravations, or any serious adverse events were reported from either group.
The authors concluded that there was a small, but non-significant direction of effect favoring homeopathy against placebos in treatment of post-stroke hemiparesis.
Considering the fact that homeopathy has become the holy cow of India which led to the phenomenon that almost no negative homeopathy trials are being reported by Indian researchers, this article is a happy surprise. Its authors clearly report that IHM had no effect on the primary outcome measure.
Bravo!
But who had the bizarre idea that it might?
I have heard many outlandish claims by homeopaths but the one about PSH was a new one to me.
Equally puzzling is, in my view, the design of this study: it was an “open-label, randomized, placebo-controlled trial”. The reason for having a placebo group is to blind the patients, i.e. not let them know whether they receive the verum or the placebo. In an open-label trial, however, the patient is given exactly that information. I totally fail to understand the logic of this. Can someone enlighten me, please?
Atopic dermatitis (AD) is a common condition that often frustrates all attempts of treatment. This is an ideal situation for homeopaths who claim to have the solution. Yet the evidence fails to support their optimism. The two systematic reviews on the subject are not encouraging:
- There was insufficient evidence to make recommendations on maternal allergen avoidance for disease prevention, oral antihistamines, Chinese herbs, dietary restriction in established atopic eczema, homeopathy, house dust mite reduction, massage therapy, hypnotherapy, evening primrose oil, emollients, topical coal tar and topical doxepin.
- The evidence from controlled clinical trials therefore fails to show that homeopathy is an efficacious treatment for eczema.
But now, a new study has emerged and it seems to contradict the previous conclusions. This study compared the efficacy of individualized homeopathic medicines (IHMs) against placebos in the treatment of AD.
In this double-blind, randomized, placebo-controlled trial of 6 months duration (n = 60), adult patients were randomized to receive either IHMs (n = 30) or identical-looking placebos (n = 30). All participants received concomitant conventional care, which included the application of olive oil and maintaining local hygiene. The primary outcome measure was disease severity using the Patient-Oriented Scoring of Atopic Dermatitis (PO-SCORAD) scale; secondary outcomes were the Atopic Dermatitis Burden Scale for Adults (ADBSA) and Dermatological Life Quality Index (DLQI) – all were measured at baseline and every month, up to 6 months. Group differences were calculated on the intention-to-treat sample.
After 6 months of intervention, inter-group differences became statistically significant on PO-SCORAD, the primary outcome (−18.1; 95% confidence interval, −24.0 to −12.2), favoring IHMs against placebos (F 1, 52 = 14.735; p <0.001; two-way repeated measures analysis of variance). Inter-group differences for the secondary outcomes favored homeopathy, but were overall statistically non-significant (ADBSA: F 1, 52 = 0.019; p = 0.891; DLQI: F 1, 52 = 0.692; p = 0.409).
The authors concluded that IHMs performed significantly better than placebos in reducing the severity of AD in adults, though the medicines had no overall significant impact on AD burden or DLQI.
I was unable to access the full paper, or more precisely unwilling to pay for it (in case someone has access, please post the link in the comments section below). From what can be gleaned from the abstract, this study is rigorous and clearly reported.
So, why is the outcome positive?
Pehaps one clue lies in the origin of the study. Here are the affiliations of the authors:
- 1Department of Materia Medica, Mahesh Bhattacharyya Homoeopathic Medical College and Hospital, Howrah, West Bengal, India.
- 2Department of Pathology and Microbiology, D. N. De Homoeopathic Medical College and Hospital, Govt. of West Bengal, Kolkata, West Bengal, India.
- 3Department of Pathology and Microbiology, Mahesh Bhattacharyya Homoeopathic Medical College and Hospital, Govt. of West Bengal, Howrah, West Bengal, India.
- 4Department of Repertory, JIMS Homoeopathic Medical College and Hospital, Shamshabad, Telangana, India.
- 5Department of Repertory, Mahesh Bhattacharyya Homoeopathic Medical College and Hospital, Govt. of West Bengal, Howrah, West Bengal, India.
- 6Department of Health and Family Welfare, Homoeopathic Medical Officer, Rajganj State Homoeopathic Dispensary, Rajganj Government Medical College and Hospital, Uttar Dinajpur, West Bengal, India.
- 7Department of Pathology and Microbiology, National Tuberculosis Elimination Program Wing, Imambara Sadar Hospital, Hooghly, Govt. of West Bengal, India.
- 8Department of Organon of Medicine and Homoeopathic Philosophy, D. N. De Homoeopathic Medical College and Hospital, Govt. of West Bengal, Kolkata, West Bengal, India.
- 9Department of Repertory, The Calcutta Homoeopathic Medical College and Hospital, Govt. of West Bengal, Kolkata, West Bengal, India.
- 10Department of Health and Family Welfare, East Bishnupur State Homoeopathic Dispensary, Chandi Daulatabad Block Primary Health Centre, Govt. of West Bengal, India.
- 11Department of Repertory, D. N. De Homoeopathic Medical College and Hospital, Kolkata, West Bengal, India.
I have previously noted that Indian studies of homeopathy (almost) never report a negative result. Why? Are the Indian homeopaths better than those elsewhere, or are they just less honest?
Aromatherapy is popular yet it has a problem: there is no indication for it. Yes, it can make you feel better but this is hardly a true medical indication. I know of many things that make me feel better, and I would not call them a THERAPY! But perhaps this new study from Iran offers a solution for the dilemna:
Sleep plays an essential role in infant development. This randomized clinical trial investigated the effect of aromatherapy with rose water on the deep sleep status of premature infants admitted to a neonatal intensive care unit (NICU).
The study was conducted on 64 infants hospitalized in NICUs. In the intervention group, two drops of rose water were poured on gas and placed next to the babies’ heads. The control group was treated in the same way except that distilled water was employed. The ALS scale was used to assess the sleep status.
Of the 66 infants in this study, 30 were female and 36 were male. The average gestational age of the infants was 32.5 ± 1.99 weeks. The results showed that the amount of deep sleep (type A and B) in the intervention group was significantly higher than the control group during and after the intervention (p=0.001).
The authors concluded that, considering the positive impact of rose water in improve of sleep quality in premature babies; it can be used to improve sleeping condition of infants in hospitals, along with main treatment.
The study has many flaws and it is badly written. Yet, I find it interesting. If its results can be confirmed with a more rigorous trial, aromatherapy might finally find a true medical purpose.
Yesterday, it has been reported that Indian scientists found the mode of action of homeopathic remedies. This is the newspaper article:
And this seems to be the abstract of the actual paper:
Homeopathic medicines contain ultra-low concentrations of metal and compounds, and it is challenging to classify homeopathic potencies using modern characterization tools. This work presents a novel experimental tool for classifying various homeopathic medicines under a low-frequency generated electromagnetic (EM) fields. A custom-built primary coil is used for generating EM fields at different excitation frequencies. The potentized test samples were prepared at decimal dilution scale of Ferrum with α‑lactose monohydrate and exhibited significant and distinct induced EM responses in the second sensing coil. The measured responses decrease logarithmically due to reducing Ferrum concentration. The resolution improved in higher potencies from 0.03 µV at 300 Hz to 0.24 µV at 4.8 kHz. Different compounds of homeopathic medicines were also investigated to produce distinct induced EM characteristics. These results were correlated with Raman spectroscopy, impedance analyser, and FT-IR analysis. The experimental investigation confirmed the classification of potencies and the technique developed to detect ultra-low metallic concentrations.
I might be a bit slow on the uptake – but I don’t see how this investigation proves anything. Perhaps someone can explain it to me?
I have grumbled about prevalence surveys in so-called alternative medicine (SCAM) before, I know. But, as the problem continues to get on my nerves – I estimate that there are about 10 times more surveys in SCAM than in any other field – allow me to do it again. The subject appeared on my screen in the form of a recent article from a minor, not Medline-listed journal. The paper is entitled:
Investigation of Complementary and Alternative Medicine Use in Turkish Patients with Epilepsy
This type of prevalence survey is typical of its genre and stands for hundreds – thousands even – like it. Its findings reveal a high prevalence of use. From that result, enthusiasts tend to draw stereotypical conclusions, namely that we need more research and that we ought to consider the integration of SCAM into routine care.
WHAT A WASTE OF TIME AND EFFORT!
Who really needs to know how many epilepsy patients in Turkey use SCAM?
Nobody!
You disagree?
Fine, then tell me: why Turkey and why epilepsy? If such information were important (and the methodology of the survey were perfect [which it hardly ever is]), then we surely need it for all diseases. How many different diseases are there? Let’s make it easy and say 1000. This means we need 1000 surveys to obtain a valuable picture of SCAM use in Turkey.
And if this sort of information is relevant in Turkey, we need to have it also for all other major countries. How many major countries exist? Let’s make it simple again and say 500. This means that we need 500 x 1000 or 500 000 surveys to generate a meaningful picture of SCAM use.
Since SCAM use changes quickly, we require these articles to be updated regularly; let’s say every 3 years. That means we require half a million surveys every 3 years.
What for?
What would it tell us?
What would we conclude from this enormous body of research?
Yes, of course, we would conclude that we need more research and we ought to consider the integration of SCAM into routine care!
My point is that if we truly need more research, why not get on with it? Why not finally forget about such useless surveys and do the science? Why not determine which SCAM works for what condition and at what risks? And, in case the findings turn out to be positive [but only then], let’s talk about integration into routine care. To put it even blunter:
The survey mania in SCAM prevents progress.
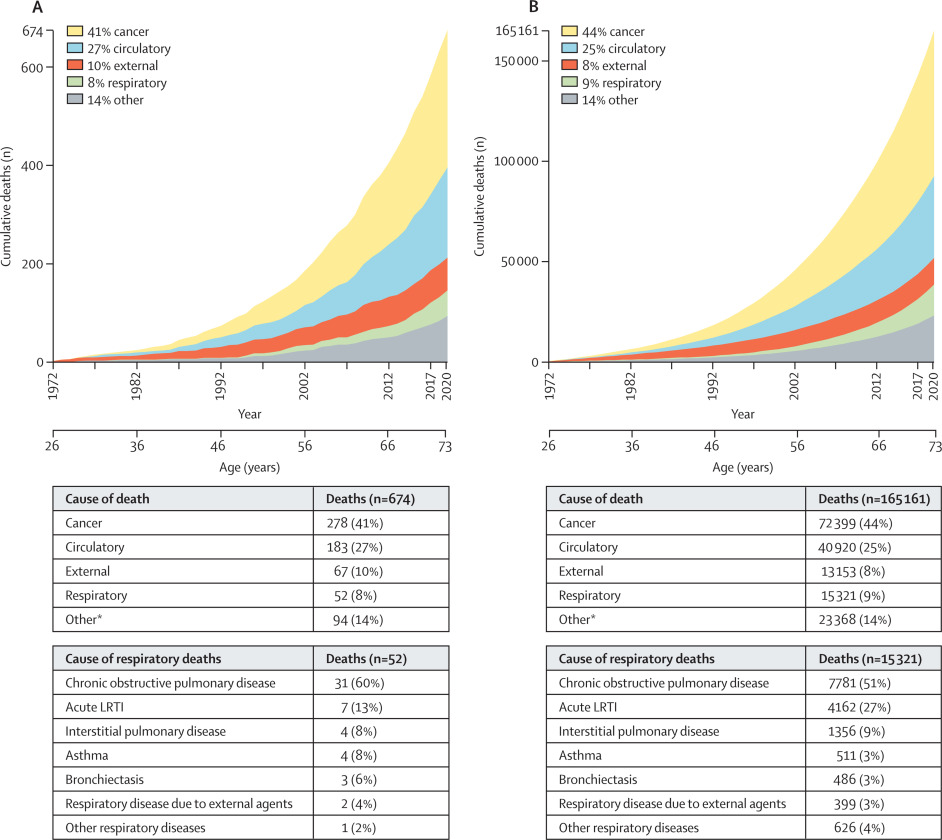
Lower respiratory tract infections (LRTIs) in early childhood are known to influence lung development and lifelong lung health, but their link to premature adult death from respiratory disease is unclear. This analysis aimed to estimate the association between early childhood LRTI and the risk and burden of premature adult mortality from respiratory disease.
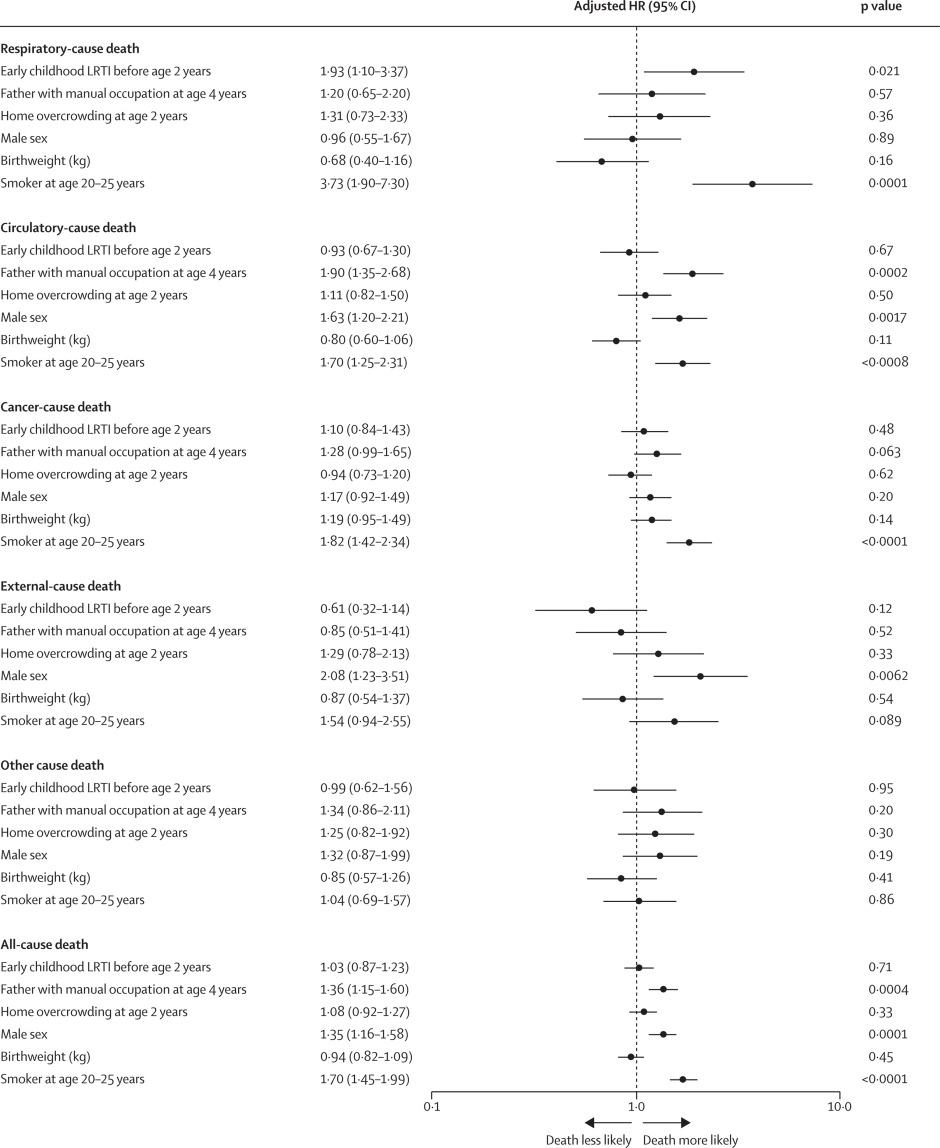
This longitudinal observational cohort study used data collected prospectively by the Medical Research Council National Survey of Health and Development in a nationally representative cohort recruited at birth in March 1946, in England, Scotland, and Wales. It evaluated the association between LRTI during early childhood (age <2 years) and death from respiratory disease from age 26 through 73 years. Early childhood LRTI occurrence was reported by parents or guardians. Cause and date of death were obtained from the National Health Service Central Register. Hazard ratios (HRs) and population attributable risk associated with early childhood LRTI were estimated using competing risks Cox proportional hazards models, adjusted for childhood socioeconomic position, childhood home overcrowding, birthweight, sex, and smoking at age 20–25 years. The researchers compared mortality within the cohort studied with national mortality patterns and estimated corresponding excess deaths occurring nationally during the study period.
5362 participants were enrolled in March, 1946, and 4032 (75%) continued participating in the study at age 20–25 years. 443 participants with incomplete data on early childhood (368 [9%] of 4032), smoking (57 [1%]), or mortality (18 [<1%]) were excluded. 3589 participants aged 26 years (1840 [51%] male and 1749 [49%] female) were included in the survival analyses from 1972 onwards. The maximum follow-up time was 47·9 years. Among 3589 participants, 913 (25%) who had an LRTI during early childhood were at greater risk of dying from respiratory disease by age 73 years than those with no LRTI during early childhood (HR 1·93, 95% CI 1·10–3·37; p=0·021), after adjustment for childhood socioeconomic position, childhood home overcrowding, birthweight, sex, and adult smoking. This finding corresponded to a population attributable risk of 20·4% (95% CI 3·8–29·8) and 179 188 (95% CI 33 806–261 519) excess deaths across England and Wales between 1972 and 2019.
The authors concluded that, in this perspective, life-spanning, nationally representative cohort study, LRTI during early childhood was associated with almost a two times increased risk of premature adult death from respiratory disease, and accounted for one-fifth of these deaths.
What has that got to do with so-called alternative medicine?
Nothing!
Yet, I feel that this study is so remarkable that I need to report on it nonetheless.
What do the findings indicate?
I am not sure. Perhaps they confirm that our genetic makeup is hugely important in determining our health. Thus even the earliest signs of weakness can provide an indication of what might happen in later life.
Whatever the meaning, I find this study fascinating and hope you agree.