politics
In the UK – this post is mainly for UK readers – journalists and opinion leaders are currently falling over themselves reporting about a major breakthrough: an Alzheimer’s drug has been shown to slow the disease by around 36%. “After 20 years with no new Alzheimer’s disease drugs in the UK, we now have two potential new drugs in 12 just months,” wrote Dr Richard Oakley, associate director at the Alzheimer’s Society. And the Daily Mail headlined: “New drug which claims to slow mental decline caused by Alzheimer’s by 36% could spell ‘the beginning of the end’ for the degenerative brain disease”.
That’s excellent news!
Many people will have made a sigh of relief!
So, why does it make me angry?
Once we listen to the news more closely we learn that:
- the drug only works for patients who are diagnosed early;
- for an early diagnosis, we need a PET scan;
- the UK hardly has any PET scanners, in fact, we have the lowest number among developed countries;
- these scanners are very expensive;
- the costs for the new drug are as yet unknown but will also be high.
Collectively these facts mean that we have a major advance in healthcare that could help many patients. At the same time, we all know that this is mere theory and that the practice will be very different.
Why?
- Because the NHS has been run down and is on its knees.
- Because our government will again say that they have invested xy millions into this area.
- The statement might be true or not, but in any case, the funds will be far too little.
- The UK has become a country where some patients suffering from severe toothache currently resort to pulling out their own teeth at home with pairs of pliers.
- In the foreseeable future, the NHS will not be allocated the money to invest in sufficient numbers of PET scans (not to mention the funds to buy the new and expensive drug).
In other words, the UK celebrates yet another medical advance raising many people’s expectations, while everyone in the know is well aware of the fact that the UK public will not benefit from it.
Does that not make you angry too?
It been reported that the German HEILPRAKTIKER, Holger G. has been sentenced to serve a total of 4 years and three months behind bars. He made himself a pair of glasses out of aluminum foil and appeared at the start of his trial wearing a Corona protective mask. The accusations against him were fierce: He was accused of having issued false Corona vaccination certificates en masse in Munich and of having given medication to patients. A woman, who had contracted Corona and had been treated by Holger G. with vitamin solutions, had died last year.
According to the verdict, Holger G. had violated the German Medicines Act. The court announced he was also convicted of 96 counts of dangerous bodily harm and 102 counts of unauthorized trading in prescription drugs. In addition, the court ordered the HEILPRAKTIKER to be placed in a rehab facility.
The 71-year-old MAN had issued Corona vaccination cards since April 2021, without actually vaccinating the people concerned. For the forged vaccination cards, he charged several tens of thousands of Euros. In addition, the former HEILPRAKTIKER illegally sold prescription drugs. The judgment is so severe because Holger G. has form. He also ordered to bear the costs of the proceedings.
___________________________
I have long criticized the German HEILPRAKTIKER. In my recent book on the subject, I make the following points:
– Today, no one can provide reliable data on the number of HEILPRAKTIKER in Germany.
– The training of HEILPRAKTIKER is woefully inadequate.
– The far-reaching rights of the HEILPRAKTIKER are out of proportion to their overt lack of competence.
– This disproportion poses a serious danger to patients.
– This danger is further increased by the fact that there is no effective control of the activity of the HEILPRAKTIKER does not take place.
– Existing laws are almost never applied to the HEILPRAKTIKER.
– Most HEILPRAKTIKER mislead the public unhindered with untenable therapeutic claims.
– The federal government seems to put off over and over again any serious discussion of the HEILPRAKTIKER.
Cases like the one above show that it is high time for reform – or, should that prove impossible, the discontinuation of this utterly obsolete and highly dangerous profession.
“We are hugely concerned about the welfare of doctors and healthcare workers with long COVID”. These are the first words of a comprehensive survey of UK doctors with post-acute COVID health complications. It reveals that these doctors experience symptoms such as:
- fatigue,
- headaches,
- muscular pain,
- nerve damage,
- joint pain,
- respiratory problems.
Around 60% of doctors said that post-acute COVID ill health has affected their ability to carry out day-to-day activities on a regular basis. 18% reported that they were now unable to work due to their post-acute COVID ill-health, and only 31% said they were working full-time, compared with more than half before the onset of their illness.
The report demands financial support for doctors and healthcare staff with post-acute COVID, post-acute COVID to be recognized as an occupational disease in healthcare workers, with a definition that covers all of the debilitating disease’s symptoms and for improved access to physical and mental health services to aid comprehensive assessment, appropriate investigations and treatment. The report also calls for greater workplace protection for healthcare staff risking their lives for others and better support for post-acute COVID sufferers to return to work safely if they can, including a flexible approach to the use of workplace adjustments.
In November 2021, an online survey investigating the emotional states of depression, anxiety, stress, compassion satisfaction, and compassion fatigue was administered to 78 Italian healthcare workers (HCWs). Between 5 and 20% of the cohort showed the effects of the adverse psychological impact of the pandemic and more than half of them experienced medium levels of compassion fatigue as well as a medium level of compassion satisfaction. The results also show that those with fewer years of clinical practice might be at greater risk of burnout, anxiety, and stress symptoms and might develop a lower level of compassion satisfaction. Moreover, the factors that potentially contribute to poor mental health, compassion fatigue, and compassion satisfaction seem to differ between residents and specialist physicians.
A cross-sectional study was conducted from September 2021 to April 2022 and targeted all physicians working at King Fahd Hospital of the University, Al Khobar, Saudi Arabia. Patient Health Questionnaire-9 and General Anxiety Disorder-7 were used to elicit self-reported data regarding depression and anxiety, respectively. In addition, sociodemographic and job-related data were collected. A total of 438 physicians responded, of which 200 (45.7%) reported symptoms of depression and 190 (43.4%) of anxiety. Being aged 25-30 years, female, resident, and reporting a reduction in work quality were factors significantly associated with both anxiety and depression. Female gender (AOR = 3.570; 95% CI = 2.283-5.582; P < 0.001), working an average 9-11 hours/day (AOR = 2.130; 95% CI = 1.009-4.495; P < 0.047), and self-perceived reduction in work quality (AOR = 3.139; 95% CI = 2.047-4.813; P < 0.001) were significant independent predictors of anxiety. Female gender (AOR = 2.929; 95% CI = 1.845-4.649; P < 0.001) and self-perceived reduction in work quality (AOR = 3.141; 95% CI = 2.053-4.804; P < 0.001) were significant independent predictors of depression.
An observational, multicenter cross-sectional study was conducted at eight tertiary care centers in India. The consenting participants were HCWs between 12 and 52 weeks post-discharge after COVID-19 infection. The mean age of the 679 eligible participants was 31.49 ± 9.54 years. The overall prevalence of COVID sequelae was 30.34%, with fatigue (11.5%) being the most common followed by insomnia (8.5%), difficulty in breathing during activity (6%), and pain in joints (5%). The odds of having any sequelae were significantly higher among participants who had moderate to severe COVID-19 (OR 6.51; 95% CI 3.46-12.23) and lower among males (OR 0.55; 95% CI 0.39-0.76). Besides these, other predictors for having sequelae were age (≥45 years), presence of any comorbidity (especially hypertension and asthma), category of HCW (non-doctors vs doctors), and hospitalization due to COVID-19.
Such data are scary. Not only will we have a tsunami of long-Covid patients from the general public, and not only do we currently lack effective causal treatments for the condition, but also is the number of HCWs who are supposed to deal with all this drastically reduced.
Most if not all countries are going to be affected by these issues. But the UK public might suffer the most, I fear. The reasons are obvious if you read a previous post of mine: in the UK, we have significantly fewer doctors, nurses, hospital beds, and funding (as well as politicians who care and would be able to do something about the problem) than in other comparable countries. To me, this looks like the emergence of a perfect storm.
This review assessed the role of homoeopathy in the therapeutic management of substance use disorders (SUD) through a systematic web-based literature search. A comprehensive search was conducted online and manually to identify homoeopathic research studies published between 1993 and 2022 on SUD in international databases and the Central Council of Research in Homoeopathy library. Relevant studies were categorised and assessed in terms of study designs, number of participants, evidence grades and clinical outcome parameters. A total of 21 full-text studies were screened and evaluated. Risk of bias (RoB) was assessed for all studies and model validity was appraised for the included RCTs’.
10 studies were included:
- 3 Randomised Controlled Trials,
- 3 Observational studies,
- 1 Pilot study,
- 1 observational comparative study,
- 1 retrospective cohort study,
- 1 case series.
Three studies have a level of evidence of 1b with an ‘A’ grade of recommendation, which consists of the RCTs only. The most commonly prescribed medicines identified were:
- Arsenic album,
- Nux vomica,
- Lycopodium,
- Pulsatilla,
- Sulphur,
- Staphysagria,
- Belladonna,
- Ipecac,
- Chamomilla,
- Rhustox,
- Phosphorus,
- Lachesis.
A high risk of bias was elicited in most of the observational studies accentuating the need for more robust methodological studies.
The authors concluded that the majority of the studies have a small number of recruitments. Pragmatic studies with larger sample sizes and validated outcome measures may be designed further to validate the
promising role of homoeopathic medicines in SUDs and generate quality evidence.
The paper is surprising! Most of the studies are not RCTs and thus cannot come even near suggesting a causal effect of homeopathy. The three RCTs are the following:
- Manchanda RK, Janardanan Nair KR, Varanasi R, Oberai P, Bhuvaneswari R, Bhalerao R, et al. A randomised comparative trial in the management of alcohol dependence: Individualised homoeopathy versus standard allopathic treatment. Indian J Res Homoeopathy; 2016.
- Adler UC, Acorinte AC, Calzavara FO, et al. Double-blind evaluation of homeopathy on cocaine craving: A randomised controlled pilot study. J Integr Med. 2018; 16(3):178-184.
- Grover A, Bhushan B, Goel R. Double-blind placebo-controlled trial of homoeopathic medicines in the
management of withdrawal symptoms in opium addicts and its alkaloid derivatives dependents. Indian J Res Homoeopathy. 2009;3:41-4.
All of these 3 studies were assessed by the review authors as having major flaws. Only one is available on Medline:
Background: Brazil is among the nations with the greatest rates of annual cocaine usage. Pharmacological treatment of cocaine addiction is still limited, opening space for nonconventional interventions. Homeopathic Q-potencies of opium and Erythroxylum coca have been tested in the integrative treatment of cocaine craving among homeless addicts, but this setting had not proven feasible, due to insufficient recruitment.
Objective: This study investigates the effectiveness and tolerability of homeopathic Q-potencies of opium and E. coca in the integrative treatment of cocaine craving in a community-based psychosocial rehabilitation setting.
Design, setting, participants, and interventions: A randomized, double-blind, placebo-controlled, parallel-group, eight-week pilot trial was performed at the Psychosocial Attention Center for Alcohol and Other Drugs (CAPS-AD), Sao Carlos/SP, Brazil. Eligible subjects included CAPS-AD patients between 18 and 65 years of age, with an International Classification of Diseases-10 diagnosis of cocaine dependence (F14.2). The patients were randomly assigned to two treatment groups: psychosocial rehabilitation plus homeopathic Q-potencies of opium and E. coca (homeopathy group), and psychosocial rehabilitation plus indistinguishable placebo (placebo group).
Main outcome measures: The main outcome measure was the percentage of cocaine-using days. Secondary measures were the Minnesota Cocaine Craving Scale and 12-Item Short-Form Health Survey scores. Adverse events were reported in both groups.
Results: The study population comprised 54 patients who attended at least one post-baseline assessment, out of the 104 subjects initially enrolled. The mean percentage of cocaine-using days in the homeopathy group was 18.1% (standard deviation (SD): 22.3%), compared to 29.8% (SD: 30.6%) in the placebo group (P < 0.01). Analysis of the Minnesota Cocaine Craving Scale scores showed no between-group differences in the intensity of cravings, but results significantly favored homeopathy over placebo in the proportion of weeks without craving episodes and the patients’ appraisal of treatment efficacy for reduction of cravings. Analysis of 12-Item Short-Form Health Survey scores found no significant differences. Few adverse events were reported: 0.57 adverse events/patient in the homeopathy group compared to 0.69 adverse events/patient in the placebo group (P = 0.41).
Conclusions: A psychosocial rehabilitation setting improved recruitment but was not sufficient to decrease dropout frequency among Brazilian cocaine treatment seekers. Psychosocial rehabilitation plus homeopathic Q-potencies of opium and E. coca were more effective than psychosocial rehabilitation alone in reducing cocaine cravings. Due to high dropout rate and risk of bias, further research is required to confirm our findings, with specific focus on strategies to increase patient retention.
This study can hardly be said to show convincing evidence for homeopathy.
This paper is all the more surprising if we consider the affiliations of the authors:
- Clinical Research Unit (H), Aizawl under Central Council for Research in Homoeopathy, Ministry of AYUSH, Govt. of India, India.
- All India Institute of Ayurveda, New Delhi, India.
- Department of Materia Medica, Madhav Homoeopathic Medical College and Hospital, Madhav Hills,
Opposite Banas River, Abu Road, Rajasthan, India.
It is time, I think, that Indian officials and researchers learn some critical thinking and formulate the conclusions of reviews based on the evidence they produced. This would be a start:
Our review has not generated convincing evidence to suggest that homeopathy is effective in treating SUDs.
Guest post by Ken McLeod
Readers will have no trouble recalling that crank ‘naturopath’ Barbara O’Neill has graced these pages several times. She is subject to a Permanent Prohibition Order by the New South Wales Health Care Complaints Commission. It goes like this:
“The Commission is satisfied that Mrs O’Neill poses a risk to the health and safety of members of the public and therefore makes the following prohibition order:
“Mrs O’Neill is permanently prohibited from providing any health services, as defined in s4 Of the Health Care Complaints Act 1993, whether in a paid or voluntary capacity.’ 1
Evidently Ms O’Neill has scrambled her chakras or muddled her meridians because she continues to forget the Order. For example;
O’Neill did a video interview concerning the Prohibition Order and that has been posted online at YouTube.2 The video was posted ‘1 year ago,’ has had 323,000 views and had 1,598 comments. She goes into great detail what she regards as the appalling treatment at the hands of the HCCC.
In the video she admits that she has continued to travel the world spreading her lies and misrepresentations. Some of the lies are that she is a naturopath, and was a nurse, and ‘I used to work in the Operating Theatre as a psychiatric nurse….’
In the video at 53:20 in the video she refers to an aboriginal man ‘Dan’ who works at her Misty Mountain Lifestyle Retreat, (note the present tense), who is in his 50s was obese and recently had a heart attack, ‘was on a lot of medications,’ ‘was a bit scared of coming off medications,’ ‘I said Dan, I think it’s time to stop your blood pressure medications, you’re going too low, you’re a 100 over 60,’ ‘three days later his blood pressure was 100 over 75,….’ 3
Call me a cynic, but that strikes me as rather dangerous advice, worthy of an investigation by the HCCC. Meanwhile, there is no sign of ‘Dan ‘ in Misty Mountain’s ‘About page.’ Dan’s brother Dave appears, but no Dan.4 Could it be that O’Neill’s advice led to some incapacity? Tips are welcome.
Meanwhile, readers could learn much more about Barbara O’Neill at Wikipedia.5
This article first appeared in the June issue of the Australian Skeptic Magazine,6 reprinted with kind permission.
REFERENCES
1 https://www.hccc.nsw.gov.au/decisions-orders/public-statements-and-warnings/public-statement-and-statement-of-decision-in-relation-to-in-relation-to-mrs-barbara-o-neill
2 https://www.youtube.com/watch?v=tsbK5TLdAPo
3 This was dangerous and reckless advice. The full transcript is here
4 https://www.mmh.com.au/our-story
5 https://en.wikipedia.org/wiki/Barbara_O%27Neill
6 https://www.skeptics.com.au/magazine/
It has been reported that a GP has been erased from the medical register after a disciplinary tribunal concluded yesterday that her statements on vaccines amounted to misconduct.
Dr Jayne Donegan, who no longer works as an NHS GP, was found by the tribunal to have ‘encouraged parents to mislead healthcare professionals about their children’s diet or immunization history’. The UK General Medical Council (GMC) brought several allegations against Dr Donegan, about statements made between 2019 and 2020, however, the determination of impaired fitness to practise (FTP) and subsequent erasure was based solely on her suggestions to parents.
The tribunal determined that her misconduct ‘posed an ongoing risk to patient safety given her lack of insight and lack of remediation’ and that ‘public confidence would be undermined’ if such a doctor was allowed to remain in practice. An immediate order of suspension was imposed, which the tribunal determined necessary for the ‘protection of the public’. Other GMC allegations, such as Dr. Donegan’s statements failing to ‘give balanced information on the risks and benefits of immunization’, were proved true by the tribunal but were not determined to be serious misconduct.
Dr. Donegan works as a homeopathic and naturopathic practitioner and has been ‘researching disease ecology and vaccination since 1994’, according to her website. The tribunal considered statements made by Dr. Donegan in a consultation with an undercover reporter and during her lectures on vaccination. She had said that the historical decline in deaths from whooping cough was because of sanitation and surgeons, not vaccinations. She had also suggested to audiences at her lectures that they could avoid answering questions from healthcare professionals about their child’s immunization history. When asked by an audience member about this, Dr. Donegan had said: ‘I thought what am I going to do because if I were you, I could just forge something but I can’t do that because I am a doctor and I would get struck off and I really would get struck off. What can I do? I thought maybe I can do something homeopathic because they are not having it. In the meantime, I wrote “Yes, I’ll get it done” thinking what will I do and they never came back to me, so when the next one went I just said “yes. The main thing is, don’t stick your head above the parapet because you make it difficult for them. If you say they are not vaccinated, they say they can’t go on the trip or they say “They could but the insurers won’t insure us”, so just keep saying “yes” but don’t say I said that.’
The tribunal concluded that comments like this made it clear Dr. Donegan was aware this was a ‘serious matter that could result in her being struck off’, despite her defense that she was simply ‘making people laugh’. The MPTS tribunal chair Mr Julian Weinberg said: ‘The Tribunal considered that honest and accurate communication of an individual’s medical history forms an essential part of ongoing patient healthcare and that any attempt to undermine this risks the safety of patients. It noted that whilst no dishonesty was found against Dr. Donegan, the Tribunal has found that she encouraged parents to be dishonest with healthcare professionals by, for example, forging medical documents/records, thereby undermined this essential quality of the doctor/patient relationship.’ Mr Weinberg highlighted that the tribunal’s findings did not concern ‘the rights or wrongs of her views on immunization’ but rather her encouragement to parents to mislead healthcare professionals.
Dr. Donegan said in response to the decision: ‘I boycotted the GMC’s political show trial against me which ended today. Serious irregularities include bogus dishonesty charges and bogus accusations that I put newborns at risk of serious harm.’ She added: ‘Being struck off by a corrupt GMC is a small price to pay for taking a lawful ethical stand for the safety of British children.’
Apparently, Dr. Donegan even claimed that she is delighted to be struck off the register of medical practitioners – and so, I presume, are many of us reading this post!
Yesterday, the NHS turned 75, and virtually all the newspapers have joined in the chorus singing its praise.
RIGHTLY SO!
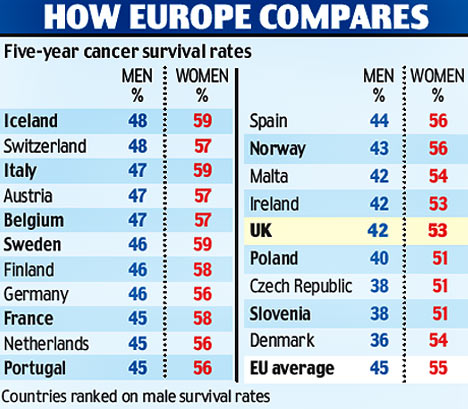
The idea of nationalized healthcare free for all at the point of delivery is undoubtedly a good one. I’d even say that, for a civilized country, it is an essential concept. The notion that an individual who had the misfortune to fall ill might have to ruin his/her livelihood to get treated is absurd and obscene to me.
The NHS was created the same year that I was born. Even though I did not grow up in the UK, I cannot imagine a healthcare system where people have to pay to get or stay healthy. To me, ‘free’ – it is, of course not free at all but merely free at the point of delivery – is a human right just as freedom of speech or the right to a good education.
 While reading some of what has been written about the NHS’s 75th birthday, I came across more platitudes than I care to remember. Yes, we are all ever so proud of the NHS but we would be even more proud if our NHS did work adequately. I find it somewhat hypocritical to sing the praise of a system that is clearly not functioning nearly as well as that of comparable European countries (where patients also don’t have to pay out of their own pocket for healthcare). I also find it sickening to listen to politicians paying lip service, while doing little to fundamentally change things. And I find it enraging to see how the conservatives have systematically under-funded the NHS, while pretending to support it adequately.
While reading some of what has been written about the NHS’s 75th birthday, I came across more platitudes than I care to remember. Yes, we are all ever so proud of the NHS but we would be even more proud if our NHS did work adequately. I find it somewhat hypocritical to sing the praise of a system that is clearly not functioning nearly as well as that of comparable European countries (where patients also don’t have to pay out of their own pocket for healthcare). I also find it sickening to listen to politicians paying lip service, while doing little to fundamentally change things. And I find it enraging to see how the conservatives have systematically under-funded the NHS, while pretending to support it adequately.
How can we be truly proud of the NHS when it seems to be dying a slow and agonizing death due to political neglect? In the UK, politicians like to be ‘world beating’ with everything, and I am sure some Tories want you to believe that, under their leadership, a world-beating healthcare system has been established in the UK.
Let me tell you: it’s not true. I have personal experience with the healthcare systems of 5 different nations and worked as a doctor in 3 of them. In Austria, France, and Germany for instance, the system is significantly better and no patient’s finances are ruined through illness.
Now there is talk about reform – yet again! Let us please not look towards the US when thinking of reforming the NHS. I have lived for a while in America and can tell you one thing: when it comes to healthcare, the US is not a civilized country. If reform of the NHS is again on the cards, let us please look towards the more civilized parts of the world!
I missed this paper when it first came out in 2022. Yet, it seems potentially quite important and I, therefore, feel like discussing it here:
President of the UNESCO Committee on Bioethics Stefan Semplici called on the governments of all countries to ensure free and wider access of their citizens to alternative medicine and pay for this therapy through health insurance. Alternative medicine based on tradition – traditional medicine, in many poor countries is the only treatment option for the population. In developed countries, and especially in China and India, it enjoys well-deserved prestige (for example, acupuncture and herbal medicine) and is often integrated into the public health system.
The International Committee on Bioethics of UNESCO announced the recognition of these alternative therapies as an option for medical practice and, at the same time, as part of the identity of the cultural traditions of various nations. The UNESCO Universal Declaration on Bioethics and Human Rights includes the right to the highest attainable standard of health (Article 14), the right to respect for pluralism and cultural diversity (Article 12) and traditional knowledge (Article 17). The purpose of this document is to establish criteria for the respect and acceptability of different types of medicine without compromising the assurance of quality and patient safety that is essential in all treatments.
In order to adapt the traditions of traditional therapies to advances in medicine, this international organization calls on governments and the scientific community to collaborate with practitioners of alternative therapies to evaluate their effectiveness and safety and develop therapeutic standards and protocols for integrating traditional medicine into healthcare system. The UNESCO International Bioethics Committee believes that these methods should be seen as complementary to modern medicine, and not just an alternative to it.
_________________________
The United Nations Educational, Scientific and Cultural Organization (UNESCO) is an agency of the United Nations aimed at promoting world peace and security through international cooperation in education, arts, sciences, and culture. UNESCO’s International Bioethics Committee (IBC) is a body of 36 independent experts that follows progress in the life sciences and its applications in order to ensure respect for human dignity and freedom.
I have to say that I rarely have seen an announcement in so-called alternative medicine (SCAM) that is more confusing and less well thought through. The UNESCO Committee on Bioethics wants:
- alternative therapies as an option for medical practice,
- the highest attainable standard of health,
- to collaborate with practitioners of alternative therapies to evaluate their effectiveness and safety.
When I first read these lines, I asked myself: who on earth wrote such nonsense? It was certainly not written by someone who understands healthcare, SCAM, and evidence-based medicine.
As discussed almost permanently on this blog, most forms of SCAM have not been shown to generate more good than harm. This means that employing them ‘as an option in medical practice’ cannot possibly produce ‘the highest attainable standards of health’. In fact, the UNESCO plan would lead to lower not higher standards. How can a committee on bioethics not realize that this is profoundly unethical?
Collaboration with practitioners of alternative therapies to evaluate SCAM’s effectiveness and safety sounds a bit more reasonable. It ignores, however, that tons of evidence already exist but fail to be positive. Why do these experts in bioethics not advocate to first make a sober assessment of the published literature?
I must say that the initiative of the UNESCO Committee on Bioethics puzzles me a lot and disturbs me even more.
I’d be keen to learn what you think of it.
The current secondary analysis based on the WHO database (VigiBase) of individual case safety reports (ICSRs) focuses on the suspected cutaneous adverse drug reactions (ADRs) linked to traditional medicines (TMs).
All the ICSRs reported between 1st January 2016 and 30th June 2021 from the UN Asia region in VigiBase where at least one TM was suspected to cause cutaneous ADRs were included in the study. Data regarding demographic details, suspected drug, adverse reaction as per MedDRA term, the seriousness of the reaction, de-challenge, re-challenge, and clinical outcome for suspected cutaneous ADRs associated with TM were obtained from VigiBase and analyzed for frequency of reported events and suspected medicines.
A total of 3,523 ICSRs with 5,761 ADRs related to “skin and subcutaneous tissue disorders” were included in the analysis. Amongst these, 6.8% of ICSRs were reported as serious.
The most common ADRs were:
- pruritus (29.6%),
- rash (20.3%),
- urticaria (18.9%),
- hyperhidrosis (3.3%).
Artemisia argyi H.Lév. and Vaniot. (14.9%), Ginkgo biloba L. (5.1%), Vitis vinifera L. (4%), Vitex agnus-castus L. (3.8%), Silybum marianum (L.), Gaertn (3.5%), and Viscus album L. (2.7%) were some commonly suspected TMs for cutaneous ADRs. There were 46 cases of Stevens-Johnson syndrome and toxic epidermal necrolysis reported with TMs during the study period. Death was reported in 5 ICSRs.
The authors concluded that TMs are linked with various cutaneous ADRS ranging from pruritus to toxic epidermal necrolysis which may have serious consequences. TMs listed as suspected offending agents in this analysis, should be kept in mind while dealing with suspected cutaneous ADRs. Clinicians should be more vigilant in detecting and reporting events associated with TMs.
Herbal remedies have a reputation for being time-tested, gentle, harmless, and benign. Reports such as this one might make us doubt this cliche. More importantly, they should force us to ask whether the remedy we are tempted to try truly does generate more good than harm. In most instances, I fear, the answer is not positive.
An article in the German publication T-online is, I think, relevant to us here on this blog. I translated part of it for you:
The suspicion of particularly serious fraud against a doctor from the German Meißen district has been substantiated. Since the beginning of the pandemic, the 66-year-old physician is said to have issued “certificates of convenience” in the thousands throughout Germany, a spokesman for the public prosecutor’s office said. In return for a payment of 25 euros, the doctor from Moritzburg is said to have issued blanket and unjustified certificates stating that the wearing of mouth and nose protection was not medically justifiable. In other cases, the physician stated an unlimited inoculation prohibition or that Corona quick tests were possible only over saliva.
After an initial search in February, the public prosecutor’s office had assumed to be dealing with merely 162 false vaccination and mask certificates. But the extent of the fraud seems to go far beyond that: The accused is now said to have taken in at least 60,000 euros with the fake certificates.
Based on further investigations, the public prosecutor’s office assumes that the medical practitioner has managed to issue false corona attestations “every minute” with so-called collective appointments. These appointments were arranged in cooperation with Heilpraktiker from all parts of Germany and partly even with funeral homes.
On Tuesday, more than 360 police officers searched 140 homes of exemption certificate holders in nine states – mainly in Bavaria. In the process, 174 incorrect Corona attestations were found. They now must face instigations into using illegal health certificates. In addition, the office of a Bavarian Heilpraktiker, as well as a further commercial area, were searched.
This is not the first time that the Moritzburg doctor has come into conflict with the law. The 66-year-old physician considers herself a ‘Reichsbuerger’ (citizen of the Reich, a right-wing extremist). She was a member of the Moritzburg shooting club, and owned eleven weapons. Because they were not all registered and several hundred rounds of ammunition were found in the house, she stood trial for the first time already in 2014.





