clinical trial
Today is Valentine’s Day, a good moment to take a critical look at some of the libido-boosters so-called alternative medicine (SCAM) has to offer. The Internet offers plenty; this website, for instance, advertises over 20 different natural (mostly botanical) products. But such sites are typically thin on evidence.

A quick Medline search locates plenty of research. Much of it seems to be on rats which is not so relevant – unless, of course, your husband is a rat. In terms of clinical trials, Medline too is not all that informative. Here are some of the studies I found:
Eurycoma longifolia is reputed as an aphrodisiac and remedy for decreased male libido. A randomized, double-blind, placebo controlled, parallel group study was carried out to investigate the clinical evidence of E. longifolia in men. The 12-week study in 109 men between 30 and 55 years of age consisted of either treatment of 300 mg of water extract of E. longifolia (Physta) or placebo. Primary endpoints were the Quality of Life investigated by SF-36 questionnaire and Sexual Well-Being investigated by International Index of Erectile Function (IIEF) and Sexual Health Questionnaires (SHQ); Seminal Fluid Analysis (SFA), fat mass and safety profiles. Repeated measures ANOVA analysis was used to compare changes in the endpoints. The E. longifolia (EL) group significantly improved in the domain Physical Functioning of SF-36, from baseline to week 12 compared to placebo (P = 0.006) and in between group at week 12 (P = 0.028). The EL group showed higher scores in the overall Erectile Function domain in IIEF (P < 0.001), sexual libido (14% by week 12), SFA- with sperm motility at 44.4%, and semen volume at 18.2% at the end of treatment. Subjects with BMI ≥ 25 kg/m(2) significantly improved in fat mass lost (P = 0.008). All safety parameters were comparable to placebo.
Yoga is a popular form of complementary and alternative treatment. It is practiced both in developing and developed countries. Use of yoga for various bodily ailments is recommended in ancient ayvurvedic (ayus = life, veda = knowledge) texts and is being increasingly investigated scientifically. Many patients and yoga protagonists claim that it is useful in sexual disorders. We are interested in knowing if it works for patients with premature ejaculation (PE) and in comparing its efficacy with fluoxetine, a known treatment option for PE. Aim: To know if yoga could be tried as a treatment option in PE and to compare it with fluoxetine. Methods: A total of 68 patients (38 yoga group; 30 fluoxetine group) attending the outpatient department of psychiatry of a tertiary care hospital were enrolled in the present study. Both subjective and objective assessment tools were administered to evaluate the efficacy of the yoga and fluoxetine in PE. Three patients dropped out of the study citing their inability to cope up with the yoga schedule as the reason. Main outcome measure: Intravaginal ejaculatory latencies in yoga group and fluoxetine control groups. Results: We found that all 38 patients (25-65.7% = good, 13-34.2% = fair) belonging to yoga and 25 out of 30 of the fluoxetine group (82.3%) had statistically significant improvement in PE. Conclusions: Yoga appears to be a feasible, safe, effective and acceptable nonpharmacological option for PE. More studies involving larger patients could be carried out to establish its utility in this condition.
Antidepressants including selective serotonin reuptake inhibitors (SSRIs) and serotonin noradrenaline reuptake inhibitors (SNRIs) are known to cause secondary sexual dysfunction with prevalence rates as high as 50%-90%. Emerging research is establishing that acupuncture may be an effective treatment modality for sexual dysfunction including impotence, loss of libido, and an inability to orgasm. Objectives: The purpose of this study was to examine the potential benefits of acupuncture in the management of sexual dysfunction secondary to SSRIs and SNRIs. Subjects: Practitioners at the START Clinic referred participants experiencing adverse sexual events from their antidepressant medication for acupuncture treatment at the Mood and Anxiety Disorders, a tertiary care mood and anxiety disorder clinic in Toronto. Design: Participants received a Traditional Chinese Medicine assessment and followed an acupuncture protocol for 12 consecutive weeks. The acupuncture points used were Kidney 3, Governing Vessel 4, Urinary Bladder 23, with Heart 7 and Pericardium 6. Participants also completed a questionnaire package on a weekly basis. Outcomes measured: The questionnaire package consisted of self-report measures assessing symptoms of depression, anxiety, and various aspects of sexual function. Results: Significant improvement among male participants was noted in all areas of sexual functioning, as well as in both anxiety and depressive symptoms. Female participants reported a significant improvement in libido and lubrication and a nonsignificant trend toward improvement in several other areas of function. Conclusions: This study suggests a potential role for acupuncture in the treatment of the sexual side-effects of SSRIs and SNRIs as well for a potential benefit of integrating medical and complementary and alternative practitioners.
The primary objectives were to compare the efficacy of extracts of the plant Tribulus terrestris (TT; marketed as Tribestan), in comparison with placebo, for the treatment of men with erectile dysfunction (ED) and with or without hypoactive sexual desire disorder (HSDD), as well as to monitor the safety profile of the drug. The secondary objective was to evaluate the level of lipids in blood during treatment. Participants and design: Phase IV, prospective, randomized, double-blind, placebo-controlled clinical trial in parallel groups. This study included 180 males aged between 18 and 65 years with mild or moderate ED and with or without HSDD: 90 were randomized to TT and 90 to placebo. Patients with ED and hypertension, diabetes mellitus, and metabolic syndrome were included in the study. In the trial, an herbal medicine intervention of Bulgarian origin was used (Tribestan®, Sopharma AD). Each Tribestan film-coated tablet contains the active substance Tribulus terrestris, herba extractum siccum (35-45:1) 250mg which is standardized to furostanol saponins (not less than 112.5mg). Each patient received orally 3×2 film-coated tablets daily after meals, during the 12-week treatment period. At the end of each month, participants’ sexual function, including ED, was assessed by International Index of Erectile Function (IIEF) Questionnaire and Global Efficacy Question (GEQ). Several biochemical parameters were also determined. The primary outcome measure was the change in IIEF score after 12 weeks of treatment. Complete randomization (random sorting using maximum allowable% deviation) with an equal number of patients in each sequence was used. This randomization algorithm has the restriction that unequal treatment allocation is not allowed; that is, all groups must have the same target sample size. Patients, investigational staff, and data collectors were blinded to treatment. All outcome assessors were also blinded to group allocation. Results: 86 patients in each group completed the study. The IIEF score improved significantly in the TT group compared with the placebo group (Р<0.0001). For intention-to-treat (ITT) there was a statistically significant difference in change from baseline of IIEF scores. The difference between TT and placebo was 2.70 (95% CI 1.40, 4.01) for the ITT population. A statistically significant difference between TT and placebo was found for Intercourse Satisfaction (p=0.0005), Orgasmic Function (p=0.0325), Sexual Desire (p=0.0038), Overall Satisfaction (p=0.0028) as well as in GEQ responses (p<0.0001), in favour of TT. There were no differences in the incidence of adverse events (AEs) between the two groups and the therapy was well tolerated. There were no drug-related serious AEs. Following the 12-week treatment period, significant improvement in sexual function was observed with TT compared with placebo in men with mild to moderate ED. TT was generally well tolerated for the treatment of ED.
What makes me suspicious about these trials is that:
- they are mostly on the flimsy side,
- there are as good as no independent replications,
- they all report positive outcomes. I was unable to find a single study where the authors concluded: SORRY, BUT THIS STUFF IS USELESS!
Disappointed with the quality and the content of the existing trials, I am now off to buy some oysters!
The aim of this study was to determine the short-term effectiveness of thoracic manipulation when compared to sham manipulation for individuals with low back pain (LBP).
Patients with LBP were stratified based on symptom duration and randomly assigned to a thoracic manipulation or sham manipulation treatment group. Groups received 3 visits that included manipulation or sham manipulation, core stabilization exercises, and patient education. Three physical therapists with an average of 6 years’ experience administered the treatments according to a standardised protocol. Factorial repeated-measures analysis of variance and multiple regression were performed for pain, disability, and fear avoidance.
Ninety participants completed the study. The overall group-by-time interaction was not significant for the Modified Oswestry Disability Questionnaire, numeric pain-rating scale, and Fear-Avoidance Beliefs Questionnaire outcomes. The global rating of change scale was not significantly different between groups.

The authors concluded that three sessions of thoracic manipulation, education, and exercise did not result in improved outcomes when compared to a sham manipulation, education, and exercise in individuals with chronic LBP. Future studies are needed to identify the most effective management strategies for the treatment of LBP.
This study has many features that are praiseworthy. However, others are of concern. Lumping together chronic and acute back problems might be not ideal. And why study only short-term effects?
But foremost I do wonder why manipulations were carried out on the thoracic and not the lumbar spine, the region where the pain was located. The physiotherapist authors state that the effects of thoracic manipulation on adjacent regions have been widely studied, and the majority of authors cite regional interdependence as an explanation for its success. To some degree, this might make sense. Yet, most chiropractors and osteopaths will dismiss the trial and its findings arguing that they would manipulate at the site of subluxations.
Hurray, homeopaths have a new study to be jubilant about!
But how far can we trust its findings?
Let’s have a look.
The aim of this study was to evaluate the effects of homeopathy (H) as an adjunct to non-surgical periodontal therapy (NSPT) in individuals with type 2 diabetes (DMII) and chronic periodontitis (CP). Eighty individuals with CP and DM II participated in this randomized, double-blind, placebo-controlled study. They were randomly divided into two groups: control group (CG) and the test group (TG), and both groups received the NSPT. TG also received homeopathic therapy, including Berberis, Mercurius solubilis/Belladonna/Hepar sulphur and Pyrogenium, while CG received placebo, while the TG received placebos. Clinical and laboratorial examinations were evaluated at baseline and after 1, 6 and 12 months of treatment.
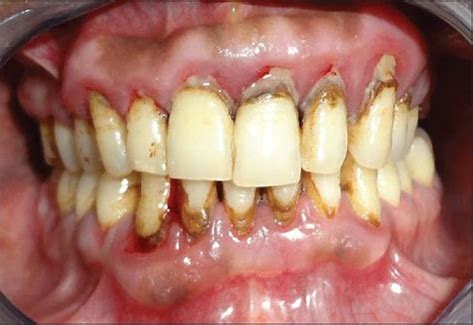
Both groups showed significant improvement throughout the study for most of the parameters studied, but TG presented a significative gain of clinical attachment at 1 and 12 months compared to CG. Mean glucose and glycated haemoglobin significantly decreased in both groups after 6 and 12 months. However, there was a significant further reduction of these parameters in TG, as compared to CG.
The authors concluded that homeopathy as supplement of NSPT may further improve health condition, including glycemic control, in DMII patients with CP.
Over the years, I have learnt how to ‘sniff out’ studies that are odd. This is one of them, I fear; it smells strangely ‘fishy’.
Here are some of the reasons why I remain sceptical:
- There does not seem to be an approval by an ethics committee.
- I also could also not find any mention of informed consent.
- There is no mention of conflicts of interest
- Neither is the source of funding disclosed.
- There were zero drop-outs which I find hard to believe.
- The trial started in 2013, but was published only recently.
- The treatment with homeopathy lacks biological plausibility.
- The authors conducted > 50 tests for statistical significance without correcting for multiple testing.
- The clinical relevance of the findings is unclear.
Even if we accepted the results of this study, we would require at least one independent replication before we allow them to influence our clinical practice.
I have been sceptical about Craniosacral Therapy (CST) several times (see for instance here, here and here). Now, a new paper might change all this:
The systematic review assessed the evidence of Craniosacral Therapy (CST) for the treatment of chronic pain. Randomized clinical trials (RCTs) assessing the effects of CST in chronic pain patients were eligible. Pain intensity and functional disability were the primary outcomes. Risk of bias was assessed using the Cochrane tool.

Ten RCTs with a total of 681 patients suffering from neck and back pain, migraine, headache, fibromyalgia, epicondylitis, and pelvic girdle pain were included.
Compared to treatment as usual, CST showed greater post intervention effects on:
- pain intensity (SMD=-0.32, 95%CI=[−0.61,-0.02])
- disability (SMD=-0.58, 95%CI=[−0.92,-0.24]).
Compared to manual/non-manual sham, CST showed greater post intervention effects on:
- pain intensity (SMD=-0.63, 95%CI=[−0.90,-0.37])
- disability (SMD=-0.54, 95%CI=[−0.81,-0.28]) ;
Compared to active manual treatments, CST showed greater post intervention effects on:
- pain intensity (SMD=-0.53, 95%CI=[−0.89,-0.16])
- disability (SMD=-0.58, 95%CI=[−0.95,-0.21]) .
At six months, CST showed greater effects on pain intensity (SMD=-0.59, 95%CI=[−0.99,-0.19]) and disability (SMD=-0.53, 95%CI=[−0.87,-0.19]) versus sham. Secondary outcomes were all significantly more improved in CST patients than in other groups, except for six-month mental quality of life versus sham. Sensitivity analyses revealed robust effects of CST against most risk of bias domains. Five of the 10 RCTs reported safety data. No serious adverse events occurred. Minor adverse events were equally distributed between the groups.
The authors concluded that, in patients with chronic pain, this meta-analysis suggests significant and robust effects of CST on pain and function lasting up to six months. More RCTs strictly following CONSORT are needed to further corroborate the effects and safety of CST on chronic pain.

Robust effects! This looks almost convincing, particularly to an uncritical proponent of so-called alternative medicine (SCAM). However, a bit of critical thinking quickly discloses numerous problems, not with this (technically well-made) review, but with the interpretation of its results and the conclusions. Let me mention a few that spring into my mind:
- The literature searches were concluded in August 2018; why publish the paper only in 2020? Meanwhile, there might have been further studies which would render the review outdated even on the day it was published. (I know that there are many reasons for such a delay, but a responsible journal editor must insist on an update of the searches before publication.)
- Comparisons to ‘treatment as usual’ do not control for the potentially important placebo effects of CST and thus tell us nothing about the effectiveness of CST per se.
- The same applies to comparisons to ‘active’ manual treatments and ‘non-manual’ sham (the purpose of a sham is to blind patients; a non-manual sham defies this purpose).
- This leaves us with exactly two trials employing a sham that might have been sufficiently credible to be able to fool patients into believing that they were receiving the verum.
- One of these trials (ref 44) is far too flimsy to be taken seriously: it was tiny (n=23), did not adequately blind patients, and failed to mention adverse effects (thus violating research ethics [I cannot take such trials seriously]).
- The other trial (ref 41) is by the same research group as the review, and the authors award themselves a higher quality score than any other of the primary studies (perhaps even correctly, because the other trials are even worse). Yet, their study has considerable weaknesses which they fail to discuss: it was small (n=54), there was no check to see whether patient-blinding was successful, and – as with all the CST studies – the therapist was, of course, no blind. The latter point is crucial, I think, because patients can easily be influenced by the therapists via verbal or non-verbal communication to report the findings favoured by the therapist. This means that the small effects seen in such studies are likely to be due to this residual bias and thus have nothing to do with the intervention per se.
- Despite the fact that the review findings depend critically on their own primary study, the authors of the review declared that they have no conflict of interest.
Considering all this plus the rather important fact that CST completely lacks biological plausibility, I do not think that the conclusions of the review are warranted. I much prefer the ones from my own systematic review of 2012. It included 6 RCTs (all of which were burdened with a high risk of bias) and concluded that the notion that CST is associated with more than non‐specific effects is not based on evidence from rigorous RCTs.
So, why do the review authors first go to the trouble of conducting a technically sound systematic review and meta-analysis and then fail utterly to interpret its findings critically? I might have an answer to this question. Back in 2016, I included the head of this research group, Gustav Dobos, into my ‘hall of fame’ because he is one of the many SCAM researchers who never seem to publish a negative result. This is what I then wrote about him:
Dobos seems to be an ‘all-rounder’ whose research tackles a wide range of alternative treatments. That is perhaps unremarkable – but what I do find remarkable is the impression that, whatever he researches, the results turn out to be pretty positive. This might imply one of two things, in my view:
- all alternative therapies are effective,
- the ‘Trustworthiness Index’ of Prof Dobos is unusual.
I let my readers chose which possibility they deem to be more likely.
Yesterday, we discussed a paper concluding (amongst other things) that there are insufficient high‐quality RCTs to judge the efficacy of acupuncture for cancer‐related pain. Today, we are looking at one that overtly contradicts this verdict.
This systematic review (published in JAMA Oncology) evaluated the existing randomized clinical trials (RCTs) for evidence of the association of acupuncture and acupressure with reduction in cancer pain. Randomized clinical trials that compared acupuncture and acupressure with a sham control, analgesic therapy, or usual care for managing cancer pain were included. The primary outcome was pain intensity measured by the Brief Pain Inventory, Numerical Rating Scale, Visual Analog Scale, or Verbal Rating Scale.
A total of 17 RCTs (with 1111 patients) were included, and data from 14 RCTs (with 920 patients) were used in the meta-analysis. Seven sham-controlled RCTs (35%) were notable for their high quality, being judged to have a low risk of bias for all of their domains, and showed that real (compared with sham) acupuncture was associated with reduced pain intensity. A favourable association was also seen when acupuncture and acupressure were combined with analgesic therapy in 6 RCTs for reducing pain intensity and in 2 RCTs for reducing opioid dose. The evidence grade was moderate because of the substantial heterogeneity among studies.
The authors concluded that this systematic review and meta-analysis found that acupuncture and/or acupressure was significantly associated with reduced cancer pain and decreased use of analgesics, although the evidence level was moderate. This finding suggests that more rigorous trials are needed to identify the association of acupuncture and acupressure with specific types of cancer pain and to integrate such evidence into clinical care to reduce opioid use.
So, which of the two conclusions should we trust?
Personally, I find the JAMA paper unimpressive to the point of being suspect. Here are some of my reasons:
- About half of the primary studies are Chinese; and we have seen repeatedly that they are unreliable and report only positive results.
- Many of the trials are published in Chinese and can thus not be checked by non-Chinese readers (nor, presumably, by the experts who acted as peer-reviewers for JAMA Oncology).
- I have my doubts about the rigor of the peer-review of some of the journals that published the primary studies included in the review.
- One paper included in the review is even a mere doctoral thesis which usually is not peer-reviewed in the usual sense.
- The authors state that they included only clinical trials that compared acupuncture and acupressure with a sham control, analgesic therapy, or usual care. However, this is evidently not true; many of the studies had the infamous A+B versus B design comparing acupuncture plus a conventional therapy against the conventional therapy. As we have discussed ad nauseam on this blog, such trials cannot produce a negative finding even if ‘A’ is a placebo.
- Contrary to what the authors claim, the quality of most of the included studies was extremely poor, as far as I can see.
- One included paper which I cannot access is entitled ‘Clinical observation on 30 cases of moderate and severe cancer pain of bone metastasis treated by auricular acupressure‘. Are the review authors seriously claiming that this is an RCT?
The more I study the details of the JAMA Oncology paper, the more I feel it might be worth a complaint to the editor with a view of initiating a thorough investigation and a possible retraction.
The aim of this review is to synthesise systematic reviews (SRs) of randomised clinical trials (RCTs) evaluating the efficacy of acupuncture to alleviate chronic pain. A total of 177 reviews of acupuncture from 1989 to 2019 met the eligibility criteria. The majority of SRs found that RCTs of acupuncture had methodological shortcomings, including inadequate statistical power with a high risk of bias. Heterogeneity between RCTs was such that meta-analysis was often inappropriate.
Having (co-) authored 13 of these SRs myself, I am impressed with the amount of work that went into this synthesis. The authors should be congratulated for doing it – and for doing it well! The paper itself differentiates the findings according to various types of pain. Here I reproduce the authors’ conclusion regarding different pain entities:
- Evidence from SRs suggests that there are insufficient high‐quality RCTs to judge the efficacy of acupuncture for chronic pain associated with various medical conditions. There is no specific NICE guidance about the use of acupuncture for chronic pain conditions irrespective of aetiology or pathophysiology, although some guidance exists for specific pain conditions (see respective sections below). Guidance by NICE on chronic pain assessment and management is currently being developed (GIDNG10069) with publication expected in August 2020.
- Evidence from the SRs suggests that acupuncture prevents episodic or chronic tension‐type headaches and episodic migraine, although long‐term studies and studies comparing acupuncture with other treatment options are still required. The current NICE guidance (clinical guideline CG150) is that a course of up to 10 sessions of acupuncture over 5–8 weeks is recommended for tension‐type headache and migraine.
- The most recent evidence from a Cochrane review of 16 RCTs suggests that acupuncture is not superior to sham acupuncture for OA of the hip, although in contrast, evidence from nonCochrane reviews suggests that there is moderate‐quality evidence that acupuncture may be effective in the symptomatic relief of pain from OA of the knee. Why there should be a difference in evidence between the knee and the hip is not known. Interestingly, guidance from NICE (CG177) states: “Do not offer acupuncture for the management of osteoarthritis”.
- Evidence suggests that there are insufficient high‐quality RCTs to judge the efficacy of acupuncture for low back pain. In 2009, NICE published guidance for the management of nonspecific low back pain that recommended a course of acupuncture as part of first line treatment. This guidance produced much debate. Subsequently, NICE have updated guidance for the management of low back pain and sciatica in people over 16 (NG59) and currently recommend in Section 1.2.8 “Do not offer acupuncture for managing low back pain with or without sciatica”, even though the evidence had not significantly changed.
- Evidence from SRs suggests that dry needling acupuncture might be effective in alleviating pain associated with myofascial trigger points, at least in the short‐term, although there are insufficient high‐quality RCTs to judge the efficacy with any degree of certainty. There is no guidance from NICE on the management of myofascial pain syndrome.
- Evidence from the SRs suggests that there are insufficient high‐quality RCTs to judge the efficacy of acupuncture for cancer‐related pain and more high‐quality, appropriately designed and adequately powered studies are needed. The most recent guidance from NICE (CSG4) recognises that patients who are receiving palliative care often seek complementary therapies, but it does not specifically recommend acupuncture. It recognises that “Many studies have a considerable number of methodological limitations, making it difficult to draw definitive conclusions”.
- Evidence from SRs suggests that there are insufficient high‐quality RCTs to judge the efficacy of acupuncture for fibromyalgia pain. There is no NICE guidance on the treatment of fibromyalgia.
- Evidence from the SRs suggests that there are insufficient high‐quality RCTs to judge the efficacy of acupuncture for primary dysmenorrhea or chronic pelvic pain. There is NICE guidance on endometriosis (NG73) [200] but this does not recommend any form of Chinese medicine for this type of pelvic pain, although acupuncture is not specifically mentioned.
- Evidence from the SRs suggests that there are insufficient high‐quality RCTs to judge the efficacy of acupuncture for pain in inflammatory arthritis. There is a NICE guideline (NG100) [201] for the treatment of rheumatoid arthritis but this does not recommend acupuncture.
- Evidence from the SRs suggests that there are insufficient high‐quality RCTs to judge the efficacy of acupuncture for neuropathic pain or neuralgia. There is NICE guidance (CG173) on the management of neuropathic pain, but acupuncture is not included in the list of recommended/not recommended treatments.
- Evidence from SRs suggests that there are insufficient high‐quality RCTs to judge the efficacy of acupuncture for a variety of other painful conditions, including lateral elbow pain, shoulder pain and labour pain. There is no guidance available from NICE on the treatment of any of these conditions.
So, what should we make of all this?
Maybe I just point out two things:
- This is a most valuable addition to the literature about acupuncture. It can serve as a reference for all who are interested in an honest account of the (lack of) value of acupuncture in the management of chronic pain.
- If a therapy has been tested in hundreds of (sadly often flawed) trials and the conclusions fail to come out clearly in favour of it, it is most likely not a very effective treatment.
Until we have data to the contrary, acupuncture should not be considered to be an effective therapy for chronic pain management.
This post is dedicated to all who claim that I never discuss anything positive about so-called alternative medicine (SCAM).
Autogenic training is a therapy developed in the 1920s by the German psychiatrist Johannes Heinrich Schultz (1884 – 1970). It is an auto-hypnotic relaxation technique popular in Germany but less so other countries. (The lack of international appreciation of autogenic training might be related to Schultz’ well-documented Nazi past. In 1935, he published an essay which supported compulsory sterilization of men to eliminate hereditary illnesses. Later he was appointed deputy director of the Göring Institute in Berlin. Through this institute, he had an active role in the extermination of mentally handicapped individuals in the framework of the ‘Aktion T4’, the Nazi’s infamous euthanasia programme.)
Autogenic training consists of mental exercises using instructions directed at different parts of the body to control bodily perceptions, such as ‘my right foot feels warm’ or ‘my left arm feels heavy’. Patients tend to report an intense sense of relaxation during and after autogenic training. Autogenic training is taught in a series of lessons by a qualified instructor.
Autogenic training should be practised regularly and does not require further supervision. It is thus an inexpensive therapy. The technique is claimed to help for a range of (mostly stress-related) conditions. However, the evidence from clinical trials is scarce and, not least due to methodological problems, less than convincing.
This systematic review was conducted to evaluate the effectiveness of autogenic training on stress responses. A total 11 studies were included in a meta-analysis. They showed that autogenic training decreased anxiety and depression, and increased the high frequency of heart rate variability as well as a reduction of anxiety score by 1.37 points (n=85, SMD=-1.37: 95% CI -2.07 to -0.67), in the studies on short-term intervention targeting healthy adults.For depression, a reduction was noted of the symptom score by 0.29 point (n=327, SMD=-0.29: 95% CI -0.50 to -0.07) in the studies on long term intervention targeting the patient group.
The authors concluded that autogenic training is effective for adults’ stress management, and nurses will be able to effectively perform autogenic training programs for workers’ stress relief at the workplace.
I cannot access the full article because it was published in Korean. Nevertheless, I feel that the conclusions are probably correct.
Why?
Because I know (most of) the primary studies and three of the RCTs are my own.
(Yet, some of my critics continue to claim that I never conducted any positive studies of SCAM)
I have to admit, I only read the DAILY MAIL, if I have to (and certainly not today). This is probably why I missed this article announcing the 1st traditional Chinese medicine to be licensed in the UK.
The plant Sigesbeckia, which has an unpleasant smell, is renowned for its ability to treat aches and pains – including those caused by arthritis. It is the active ingredient in Phynova Joint and Muscle Relief Tablets, which have just been licensed by drug safety watchdog the Medicines and Healthcare Products Regulatory Agency.
The directive also made it more difficult for medicines to get a licence as it demanded they had to have been in use for 30 years, of which at least 15 years had to be in the EU. Some Western herbal medicines have managed to gain licences in a process costing thousands of pounds to verify their ingredients. But the Phynova tablets are the first traditional Chinese medicine to be approved.
Robert Miller, chief executive of Oxford-based Phynova, said he was ‘extremely proud’, adding: ‘This has come from years of working with our Chinese colleagues. ‘Britain can now benefit from having access to high quality, regulated Chinese medicines.’ He also said that the company is planning to apply for a licence for a second traditional Chinese medicine, a cold and flu remedy.
Dr Chris Etheridge, a medical herbalist and adviser to Potter’s Herbals, celebrated the ‘good news’, adding that Sigesbeckia, which is not commonly used in the West, ‘offers an alternative to those who prefer not to take non-steroidal anti-inflammatory drugs for muscle and joint pain’.
But Michael McIntyre, chairman of the European Herbal and Traditional Medicine Practitioners Association, warned that the new product demonstrates the difficulties the EU rules created for supplying herbal products safely to the public. He said it is ‘almost impossible to satisfy the licensing conditions’. He added that some people have therefore turned to the internet to buy unlicensed products, but this means they have ‘no idea whether they are safe or effective’.
How exciting!
Exciting enough to do a quick search for the evidence. Are there any clinical trials to show or suggest that this herbal remedy does anything other than filling the bank account of the manufacturer? Sadly, the answer seems to be NO! At least, I could not find a single such study (if anyone knows more, I’d be pleased to stand corrected).
Frustrated I looked at the website of the manufacturer. Here I found this:
Exclusively containing Sigesbeckia extract, Phynova Joint and Muscle Relief Tablets is a traditional herbal medicinal product used for the relief of backache, rheumatic, joint and muscle pain as well as minor sports injuries. Sigesbeckia has been used for thousands of years around the world to relieve painful joints and muscles.
Benefits
– Relief from joint & muscle pain
– Gentle on the stomach
– No known side effects
– No known drug indications or contraindications
– Can be taken with or without food
And this:
What can Sigesbeckia be used to treat?
Traditionally used for arthritic pain, rheumatic pain, back pain and sciatica. Today, Sigesbeckia can be used for;
Backache
Back pain can occur through a sprain or strain, spasms, nerve compression, herniated discs and other problems in your lower, middle and upper back.
Poor posture, lifting and stretching, sudden movements placing strain on your lower back and sports injuries, are amongst the main culprits for causing back pain.
Minor sports injuries
Minor sports injuries can be caused by an accident such as a fall or blow, not warming up properly before exercise, pushing yourself too hard and not using the appropriate equipment or perhaps poor technique.
Rheumatic and muscular pain
Common causes of rheumatic and muscle pain can be due to; tension and stress, lack of minerals, certain medication, dehydration, sprains and strains, sleep deficiency, too much physical activity and sometimes other underlying health conditions and diseases.
General aches and pains in muscles and joints
Overexertion due to a new exercise routine or from a sprain or strain can cause general aches and pains in muscles and joints. But so too can modern day busy life. The impact on our bodies can trigger aches and pains in your muscles and joints and lower your resistance to illness and disease.
The Benefit of Sigesbeckia extract
One of the benefits of Sigesbeckia extract, as used in approved licensed products, is that it has no known side effects or interactions with other medications according to the Summary of Product Characteristics (SmPC). Always check that the product you purchase is an approved Traditional Herbal Medicine Product in the UK.
In summary: Look after your joints and muscles with Sigesbeckia
Our bodies are all different, and our approach and tolerances will vary. Used for over a thousand years and known for its anti-inflammatory and mobility benefits alongside being used for joint and muscle pain; Sigesbeckia is a herbal medicine that works best when used over time.
Looking for a traditional remedy for joint and muscle relief? Why not try Sigesbeckia?
But again no sign of a clinical trial to back up this plethora of therapeutic claims. How can this be? The answer lies in the directive mentioned in the Mail article. To obtain a licence that enables the manufacturer to make therapeutic claims, a herbal remedy merely needs to demonstrate that it has been in use for 30 years, of which at least 15 years had to be in the EU.
I think I understand the intention of the directive. But I would nevertheless have thought that, 4 years after obtaining a license, the manufacturer could have conducted a study to test whether the product works. In my view this should be a moral and ethical, if not legal obligation. The ‘test of time’ is woefully insufficient and unreliable and no basis for generating progress or securing the best interests of patients.
Considering the total lack of efficacy and safety data, do you agree that the above comment by Michael McIntyre are ironic to the extreme? And do you agree that manufacturers who manage to obtain such a license should be obliged to deliver a proof of efficacy within a reasonable period of time?
Many patients with chronic pain (CP) are prescribed opioids, a situation which has led to the much-discussed opioid crisis. Integrative medicine (IM), which combines pharmacological and so-called alternative medicine (SCAM), has been proposed as a solution. Yet, the role of SCAM therapies in reducing opioid use remains unclear.
This systematic review explored the effectiveness of the IM approach or any of the SCAM therapies to reduce or cease opioid use in CP patients. Electronic searches yielded 5,200 citations. Twenty-three studies were selected. Eight studies were randomized controlled trials, seven were retrospective studies, four studies were prospective observational, three were cross-sectional, and one was quasi-experimental. The majority of the studies showed that opioid use was reduced significantly after using IM/SCAM. Cannabinoids were among the most commonly investigated approaches in reducing opioid use, followed by multidisciplinary approaches, cognitive-behavioral therapy, and acupuncture. The majority of the studies had limitations related to sample size, duration, and study design.
The authors concluded that there is a small but defined body of literature demonstrating positive preliminary evidence that the IM approach including SCAM therapies can help in reducing opioid use. As the opioid crisis continues to grow, it is vital that clinicians and patients be adequately informed regarding the evidence and opportunities for IM/SCAM therapies for CP.
I am unimpressed by this review.
And here is why:
- Because of their design, most of the included studies do not allow any conclusions about cause and effect.
- The 8 RCTs that would allow such conclusions are mostly of poor quality.
- Some of the 8 RCTs are not even what the review authors claim to be. Here is just one example:
Background: Current levels and dangers of opioid use in the U.S. warrant the investigation of harm-reducing treatment alternatives.
Purpose: A preliminary, historical, cohort study was used to examine the association between enrollment in the New Mexico Medical Cannabis Program (MCP) and opioid prescription use.
Methods: Thirty-seven habitual opioid using, chronic pain patients (mean age = 54 years; 54% male; 86% chronic back pain) enrolled in the MCP between 4/1/2010 and 10/3/2015 were compared to 29 non-enrolled patients (mean age = 60 years; 69% male; 100% chronic back pain). We used Prescription Monitoring Program opioid records over a 21 month period (first three months prior to enrollment for the MCP patients) to measure cessation (defined as the absence of opioid prescriptions activity during the last three months of observation) and reduction (calculated in average daily intravenous [IV] morphine dosages). MCP patient-reported benefits and side effects of using cannabis one year after enrollment were also collected.
Results: By the end of the 21 month observation period, MCP enrollment was associated with 17.27 higher age- and gender-adjusted odds of ceasing opioid prescriptions (CI 1.89 to 157.36, p = 0.012), 5.12 higher odds of reducing daily prescription opioid dosages (CI 1.56 to 16.88, p = 0.007), and a 47 percentage point reduction in daily opioid dosages relative to a mean change of positive 10.4 percentage points in the comparison group (CI -90.68 to -3.59, p = 0.034). The monthly trend in opioid prescriptions over time was negative among MCP patients (-0.64mg IV morphine, CI -1.10 to -0.18, p = 0.008), but not statistically different from zero in the comparison group (0.18mg IV morphine, CI -0.02 to 0.39, p = 0.081). Survey responses indicated improvements in pain reduction, quality of life, social life, activity levels, and concentration, and few side effects from using cannabis one year after enrollment in the MCP (ps<0.001).
Conclusions: The clinically and statistically significant evidence of an association between MCP enrollment and opioid prescription cessation and reductions and improved quality of life warrants further investigations on cannabis as a potential alternative to prescription opioids for treating chronic pain.
This study is evidently NOT an RCT!
Most of the 8 RCTs investigate whether SCAM is useful for weaning opioid-dependent patients off their drug. To equate this with the question whether IM/SCAM can reduce or cease opioid use in CP patients is, I think, wrong. The way to reduce opioid use in CP patients is to prescribe less opioids. And for prescribing less opioids, we need no SCAM but we need to remember what we learned in medical school: opioids are not for routine treatment of CP!
So, why do the authors of this review try to mislead us?
Could it have something to do with some of their affiliations and the bias that goes with it?
- Canadian College of Naturopathic Medicine, North York, Ontario, Canada.
- Australian Research Centre in Complementary and Integrative Medicine, University of Technology Sydney, Ultimo, Australia.
- Pacific College of Oriental Medicine, San Diego, California, USA.
What do you think?
Radiation-induced xerostomia (RIX) is a common, often debilitating, adverse effect of radiation therapy among patients with head and neck cancer. Quality of life can be severely affected, and current treatments have limited benefit. Acupuncture is often recommended, but does it work? This study was aimed at finding out whether acupuncture can prevent RIX in patients with head and neck cancer undergoing radiation therapy.
The 2-center, phase 3, randomized clinical trial compared a standard care control (SCC) with true acupuncture (TA) and sham acupuncture (SA) among patients with oropharyngeal or nasopharyngeal carcinoma who were undergoing radiation therapy in comprehensive cancer centres in the United States and China. Patients were enrolled between December 16, 2011, and July 7, 2015. Final follow-up was August 15, 2016. Analyses were conducted February 1 through 28, 2019. Either TA or SA using a validated acupuncture placebo device were performed 3 times per week during a 6- to 7-week course of radiation therapy. The primary end point was RIX, as determined by the Xerostomia Questionnaire in which a higher score indicates worse RIX, for combined institutions 1 year after radiation therapy ended. Secondary outcomes included incidence of clinically significant xerostomia (score >30), salivary flow, quality of life, salivary constituents, and role of baseline expectancy related to acupuncture on outcomes.
Of 399 patients randomized, 339 were included in the final analysis, including 112 patients in the TA group, 115 patients in the SA group, and 112 patients in the SCC group. For the primary aim, the adjusted least square mean (SD) xerostomia score in the TA group (26.6 [17.7]) was significantly lower than in the SCC group (34.8 [18.7]) (P = .001; effect size = -0.44) and marginally lower but not statistically significant different from the SA group (31.3 [18.6]) (P = .06; effect size = -0.26). Incidence of clinically significant xerostomia 1 year after radiation therapy ended followed a similar pattern, with 38 patients in the TA group (34.6%), 54 patients in the SA group (47.8%), and 60 patients in the SCC group (55.1%) experiencing clinically significant xerostomia (P = .009). Post hoc comparisons revealed a significant difference between the TA and SCC groups at both institutions, but TA was significantly different from SA only at Fudan University Cancer Center, Shanghai, China (estimated difference [SE]: TA vs SCC, -9.9 [2.5]; P < .001; SA vs SCC, -1.7 [2.5]; P = .50; TA vs SA, -8.2 [2.5]; P = .001), and SA was significantly different from SCC only at the University of Texas MD Anderson Cancer Center, Houston, Texas (estimated difference [SE]: TA vs SCC, -8.1 [3.4]; P = .016; SA vs SCC, -10.5 [3.3]; P = .002; TA vs SA, 2.4 [3.2]; P = .45).
The authors concluded that this randomized clinical trial found that TA resulted in significantly fewer and less severe RIX symptoms 1 year after treatment vs SCC. However, further studies are needed to confirm clinical relevance and generalizability of this finding and to evaluate inconsistencies in response to sham acupuncture between patients in the United States and China.
In essence this two-centre study shows that:
- real acupuncture is better than usual care, but the effect size is small and of doubtful clinical relevance;
- real acupuncture is not significantly better than sham acupuncture;
- the findings differ remarkably between the US and the Chinese centre.
I find the last point the most interesting one. We know from previous research that acupuncture studies from China are notoriously unreliable; they never report a negative result and there is evidence that data fabrication is rife in China. The new findings seems to throw more light on this notion. In the US centre, real and sham acupuncture generated practically identical results. By contrast, in the Chinese centre, real acupuncture generated significantly better results than sham. The authors offer several hypotheses to explain this remarkable phenomenon. Yet, in my view, the most likely one is that Chinese researchers are determined to show that acupuncture is effective. Thus all sorts of unconscious or even conscious biases might get introduced into such studies.
In essence, trial therefore confirms that acupuncture is little more than a theatrical placebo, particularly if we consider the US data which, in my opinion, are more trustworthy.
Lorenzo Cohen, Professor of Palliative, Rehabilitation, and Integrative Medicine and director of the Integrative Medicine Program as well as senior author of the paper unsurprisingly disagrees. He was quoted saying: “The evidence is to a point where patients should incorporate acupuncture alongside radiation treatment as a way to prevent the severity of dry mouth symptoms. I think with this study we can add acupuncture to the list for the prevention and treatment of xerostomia, and the guidelines for the use of acupuncture in the oncology setting should be revised to include this important chronic condition.”
Who do you think is closer to the truth?
