Monthly Archives: May 2019
Chiropractors, I have repeatedly found, are somewhat slow at learning lessons even from their biggest mistakes. A new paper seem to confirm my suspicion. The purpose of this study was to investigate the report by mothers of their infants’ condition before and after a trial of care provided by chiropractors in addition to ratings of satisfaction, cost of care, and reports of any adverse events. A second purpose was to report the demographic profile of infants who presented for care to 16 chiropractic clinics in the United Kingdom.
The authors prospectively collected reports by mothers of their infants’ demographic profiles and outcomes across several domains of infant behaviour and their own mental state using the United Kingdom Infant Questionnaire. Participating chiropractors were recruited through the Royal College of Chiropractors annual meeting in January 2016. A total of 15 UK clinics and the Anglo-European College of Chiropractic University College teaching clinic volunteered to participate.
In all, 2001 mothers completed intake questionnaires and 1092 completed follow-up forms. Statistically significant improvements were reported across all aspects of infant behaviour, including:
- feeding problems,
- sleep issues,
- excessive crying,
- problems with supine sleep position,
- infant pain,
- restricted cervical range of motion,
- time performing prone positioning.
Maternal ratings of depression, anxiety, and satisfaction with motherhood also demonstrated statistically significant improvement. In total, 82% reported definite improvement of their infants on a global impression of change scale. In addition, 95% reported feeling that the care was cost-effective, and 90.9% rated their satisfaction 8 or higher on an 11-point scale. Minor self-limiting side effects were reported (5.8%) but no adverse events.
The authors concluded that mothers reported that chiropractic care for their infants was effective, safe, and cost-effective. Although the observational design makes it impossible to determine efficacy, the study’s findings indicate that, on average, the changes observed by mothers were positive and may be clinically relevant.
Where to begin? Let me try to list at least some of the most obvious flaws of this study:
- The fact that mothers (where were the fathers?) reported positive outcomes is hardly surprising. After all, they chose to consult a chiropractor and paid for the treatments. Install yourself in a McDonald’s, ask customers whether they like hamburgers, and you get a similarly meaningless result.
- Cost-effectiveness is measured in Bournemouth by asking people, ‘was it worth it’? I feel like going back there and teaching them some research methodology!
- The observational design is by no means the only reason why ‘efficacy’ cannot be determined by such a study. What about the non-validated nature of the outcome measures?
- Do the researcher mean effectiveness or efficacy? And do they know the difference at all?
- What do they think is the difference between side effects and adverse events?
- Do they think they can establish safety by asking the mothers of their patients?
So, does this study show anything useful at all?
Yes, perhaps.
I think it shows that, despite all the shameful and unpleasant history that severely damaged the reputation of chiropractic in the UK and beyond, chiropractors are still treating infants for conditions for which there is ‘not a jot of evidence‘. It seems almost as though they are ‘happily promoting bogus treatments’!
The Center for Inquiry (CFI) is a non-profit educational, advocacy, and research organization headquartered based in the US. It is also home to the Richard Dawkins Foundation for Reason & Science, the Committee for Skeptical Inquiry, and the Council for Secular Humanism. The Center for Inquiry strives to foster a secular society based on reason, science, freedom of inquiry, and humanist values. The CFI has just issued a press-release about their legal action against Walmart. Here are some quotes from it:
Walmart is committing wide-scale consumer fraud and endangering the health of its customers though its sale and marketing of homeopathic medicines … the mega-retailer is deceiving consumers by making no meaningful distinction between real medicine and useless homeopathic treatments on its shelves and in its online store, misrepresenting homeopathy’s safety and efficacy.
Click here to access the official complaint (PDF).
CFI is currently engaged in a similar suit against CVS, the nation’s largest drug retailer, which was filed in June of 2018 and is still ongoing.
“Walmart sells homeopathics right alongside real medicines, in the same sections in its stores, under the same signs,” said Nicholas Little, CFI’s Vice President and General Counsel. “Searches on its website for cold and flu remedies or teething products for infants yield pages full of homeopathic junk products. It’s an incredible betrayal of customers’ trust and an abuse of Walmart’s titanic retail power.”
“Walmart can’t claim it doesn’t know that homeopathy is snake oil, because it runs its own enormous pharmacy business and make its own homeopathic products,” said Little. “So whether it’s a scientifically proven remedy like aspirin or flatly denounced junk like homeopathic teething caplets for babies, Walmart sells all of it under its in-house ‘Equate’ branding. It’s all the same to Walmart.”
Choosing homeopathic treatments to the exclusion of evidence-based medicines can result in worsened or prolonged symptoms, and in some cases, even death. Several products have been found to contain poisonous ingredients which have affected tens of thousands of adults and children in just the last few years. As recently as May 14, the Food and Drug Administration issued warnings to five manufacturers of homeopathic products for numerous safety violations.
“Despite being among the richest corporations on Earth and the largest retailer in the United States, Walmart chooses to further pad its massive wealth by tricking consumers into throwing their money away on sham medicinal products that are scientifically proven to be useless and potentially dangerous,” said Robyn Blumner, president and CEO of the Center for Inquiry. “We intend to put a stop to it.”
CFI has lobbied for tighter regulation of homeopathic products for many years, becoming the leading advocate for science-based medicine and against the proliferation of snake oil. In 2015 CFI was invited by the FDA and the Federal Trade Commission to provide expert testimony. As a result, the FTC declared in 2016 that the marketing of homeopathic products for specific diseases and symptoms is only acceptable if consumers are told: “(1) there is no scientific evidence that the product works and (2) the product’s claims are based only on theories of homeopathy from the 1700s that are not accepted by most modern medical experts.” Last year, the FDA announced a new “risk-based” policy of regulatory action against homeopathic products.
_____________________________________________________________
I have long come to the conclusion that the dangers of homeopathy and other SCAMs should best be tackled legally. The medical case against many forms of SCAM has been clear for some time. They have been published a thousand times, yet these efforts have brought little progress in practice. Politicians tend to think in terms of their chances of re-election and thus often lack the resolve to act decisively. It is therefore now largely up to the legal professions to effectively protect consumers and patients from the many types of harm SCAM can do.
My hope is therefore that the action of the CFI against Walmart will be successful. More crucially, I hope that it will become a signal for activists from other countries to consider similar actions.
Kampo, the traditional Japanese herbal medicine, developed out of traditional Chinese herbal medicine after it was introduced into Japan in the 7th century. In the early 20th century, Kampo was further influenced by modern Western medicine and science. The Kampo system is a pragmatic and simplified version of Chinese herbal medicine. Kampo medicines are standardised and not normally individualised as in Chinese herbal medicine. They are based on the symptoms of the patient, interpreted in the tradition of Kampo. Kampo diagnostic criteria consider hypofunction and hyperfunction, heat and cold, superficies and interior, and yin and yang.
Today, Kampō is fully integrated into the Japanese national health care system, and numerous Kampo preparations are registered in Japan and reimbursable from public funds. In Japan, Kampo is thus not a so-called alternative medicine (SCAM), but outside this country it clearly is.
Standardised Kampo formulas contain mixtures of herbal ingredients. They are manufactured under proper quality control. The most commonly used plants include liquorice, ginger and Chinese peony root. Most Japanese doctors routinely prescribe Kampo medicines, and most patients combine Kampo with Western medicine. Since 2002, the teaching of Kampo has been included in Japanese curricula of medical and pharmacy education. The efficacy of Kampo medicines is often less solidly documented than one would hope or expect. There is a remarkable shortage of high-quality clinical trials.
As Kampo medicines contain pharmacologically active ingredients, they can also cause adverse effects and might interact with synthetic drugs. Yet, the risks of Kampo are currently woefully under-investigated. And this is why an analysis of adverse events associated with Kampo formulations that was just published is particularly important.
The researchers obtained reports of adverse events associated with ethical Kampo formulations from the domestic adverse-event data from July 30, 2003, to March 31, 2018. Adverse events were then categorized, and the relationships between categories of adverse events and crude drugs were analysed.
There were 4,232 reported adverse events associated with Kampo. They related to liver injury, 1,193; lung injury, 1,177; pseudoaldosteronism, 889; mesenteric phlebosclerosis, 223; drug eruption, 185; and others, 565. Among events related to both liver injury and lung injury, approximately 70% were suspected to be induced by Kampo formulations containing Scutellariae Radix.
The pseudoaldosteronism-related events, which are induced by Glycyrrhizae Radix, included several events related to muscle injury, heart failure, and arrhythmia. Events related to mesenteric phlebosclerosis, believed to be induced by long-term use of Kampo formulas containing Gardeniae Fructus, increased remarkably during the study period. Among the events related to drug eruption, approximately 35% were suspected to be induced by Kampo formulations containing Ephedrae Herba.
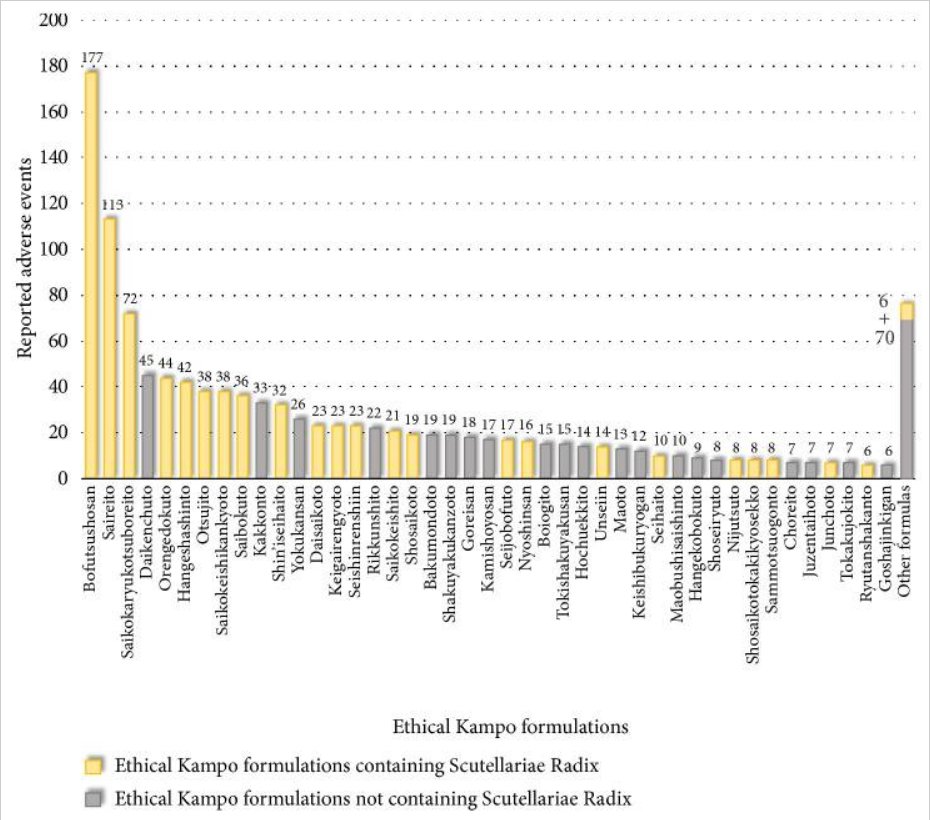 The authors concluded that Kampo medicines may cause various adverse events. The present results provide valuable information regarding adverse events associated with Kampo medicines from the viewpoint of patient safety.
The authors concluded that Kampo medicines may cause various adverse events. The present results provide valuable information regarding adverse events associated with Kampo medicines from the viewpoint of patient safety.
While this paper presents invaluable data, its authors offer little by way of critical discussion. There is hardly any good evidence to show that any of the Kampo formulas are effective. Thus a discussion needs to be had about the question whether they generate more good than harm. The authors are completely silent on this important issue, and I suspect the reason might be that Kampo is a bit of a ‘holy cow’ in Japan that must not be questioned.
The numbers of adverse events are impressively high, but we do not know how they compare to adverse events in other areas of healthcare. For instance, it would be valuable to have comparative data indicating how many adverse events occurred with Kampo compared to synthetic drugs per 1 000 patients using them.
Thus we are left with the conclusion that, once proper post-marketing methods are employed to monitor SCAM, we are likely to realise that adverse events do occur more frequently than SCAM enthusiasts might have predicted. In my view, it is high time that we have effective adverse event monitoring in all areas of SCAM.
A new paper reminds us that so-called alternative medicine (SCAM) has been increasing in the United States and around the world, particularly at medical institutions known for providing rigorous evidence-based care. The use of SCAM may cause harm to patients through interactions with prescribed medications or by patients choosing to forego evidence-based care. SCAM may also put financial strain on patients as most SCAM expenditures are paid out-of-pocket.
Despite these drawbacks, patients continue to use SCAM due to a range of reasons, e.g. media promotion of SCAM therapies, dissatisfaction with conventional healthcare, a desire for more holistic care. Given the increasing demand for SCAM, many medical institutions now offer SCAM services. Several leaders of SCAM centres based at a highly respected academic medical institution have publicly expressed anti-vaccination views, and non-evidence-based philosophies run deep within SCAM.
Although there are financial incentives for institutions to provide SCAM, it is important to recognize that this legitimizes SCAM and may cause harm to patients. The poor regulation of SCAM allows for the continued distribution of products and services that have not been rigorously tested for safety and efficacy.
As I have tried to point out many times, the potential for harm caused by the increasing integration of SCAM can thus be summarised as follows:
- direct harm due to adverse effects such as toxicity of an herbal remedy, stroke after chiropractic manipulation, pneumothorax after acupuncture;
- direct harm through the use of bogus diagnostic techniques;
- direct harm by using materials from endangered species;
- indirect harm through incompetent advice such as recommendation not to immunize or discontinue prescribed medications;
- neglect due to using SCAM instead of an effective therapy for a serious condition;
- harm due to medicalising trivial states of reduced well-being;
- financial harm due to the costs of SCAM;
- harm through making a mockery of evidence-based medicine;
- harm caused by undermining rational thinking in the society at large;
- harm caused by inhibiting medical progress and research.
In case you see other ways in which SCAM can cause harm, please let me know by posting a comment.
‘Acute-on-chronic liver failure’ (ACLF) is an acute deterioration of liver function in patients with pre-existing liver disease. It is usually associated with a precipitating event and results in the failure of one or more organs and high short term mortality.
An international team of researchers published a analysis examining data regarding drugs producing ACLF. They evaluated clinical features, laboratory characteristics, outcome, and predictors of mortality in patients with drug-induced ACLF. They identified drugs as precipitants of ACLF among prospective cohort of patients with ACLF from the Asian Pacific Association of Study of Liver (APASL) ACLF Research Consortium (AARC) database. Drugs were considered precipitants after exclusion of known causes together with a temporal association between exposure and decompensation. Outcome was defined as death from decompensation.
Of the 3,132 patients with ACLF, drugs were implicated as a cause in 10.5% of all cases and other non-drug causes in 89.5%. Within the first group, so-called alternative medications (SCAMs) were the commonest cause (71.7%), followed by combination anti-tuberculosis therapy drugs (27.3%). Alcoholic liver disease (28.6%), cryptogenic liver disease (25.5%), and non-alcoholic steatohepatitis (NASH) (16.7%) were common causes of underlying liver diseases. Patients with drug-induced ACLF had jaundice (100%), ascites (88%), encephalopathy (46.5%), high Model for End-Stage Liver Disease (MELD) (30.2), and Child-Turcotte-Pugh score (12.1). The overall 90-day mortality was higher in drug-induced (46.5%) than in non-drug-induced ACLF (38.8%).
The authors concluded that drugs are important identifiable causes of ACLF in Asia-Pacific countries, predominantly from complementary and alternative medications, followed by anti-tuberculosis drugs. Encephalopathy, bilirubin, blood urea, lactate, and international normalized ratio (INR) predict mortality in drug-induced ACLF.
The fact that some SCAMs can damage the liver has long been known. Here, for example, is 2003 our review of herbal medicine and liver problems:
Systematic literature searches were performed on Medline, Embase, The Cochrane Library, Amed and Ciscom. To identify additional data, searches were conducted by hand in relevant medical journals and in our own files. The screening and selection of articles and the extraction of data were performed independently by the two authors. There were no restrictions regarding the language of publication. In order to be included articles were required to report data on hepatotoxic events associated with the therapeutic use of herbal medicinal products.
Single medicinal herbs and combination preparations are associated with hepatotoxic events. Clinically, the spectrum ranges from transient elevations of liver enzyme levels to fulminant liver failure and death. In most instances hepatotoxic herbal constituents are believed to be the cause, while others may be due to herb-drug interactions, contamination and/or adulteration.
A number of herbal medicinal products are associated with serious hepatotoxic events. Incidence figures are largely unknown, and in most cases a causal attribution is not established. The challenge for the future is to systematically research this area, educate all parties involved, and minimize patient risk.
Despite these warnings, progress is almost non-existent. If anything the problem seems to increase in proportion with the rise in the use of SCAM. Hence, one cannot but agree with the conclusion of a more recent overview: The actual incidence and prevalence of herb-induced liver injury in developing nations remain largely unknown due to both poor pharmacovigilance programs and non-application of emerging technologies. Improving education and public awareness of the potential risks of herbals and herbal products is desirable to ensure that suspected adverse effects are formally reported. There is need for stricter regulations and pre-clinical studies necessary for efficacy and safety.
“Eating elderberries can help minimise influenza symptoms.” This statement comes from a press release by the University of Sydney. As it turned out, the announcement was not just erroneous but it also had concealed that the in-vitro study that formed the basis for the press-release was part-funded by the very company, Pharmacare, which sells elderberry-based flu remedies.
 “This is an appalling misrepresentation of this Pharmacare-funded in-vitro study,” said associate professor Ken Harvey, president of Friends of Science in Medicine. “It was inappropriate and misleading to imply from this study that an extract was ‘proven to fight flu’.” A University of Sydney spokeswoman confirmed Pharmacare was shown a copy of the press release before it was published.
“This is an appalling misrepresentation of this Pharmacare-funded in-vitro study,” said associate professor Ken Harvey, president of Friends of Science in Medicine. “It was inappropriate and misleading to imply from this study that an extract was ‘proven to fight flu’.” A University of Sydney spokeswoman confirmed Pharmacare was shown a copy of the press release before it was published.
This is an embarrassing turn of events, no doubt. But what about elderberry (Sambucus nigra) and the flu? Is there any evidence?
A systematic review quantified the effects of elderberry supplementation. Supplementation with elderberry was found to substantially reduce upper respiratory symptoms. The quantitative synthesis of the effects yielded a large mean effect size. The authors concluded that these findings present an alternative to antibiotic misuse for upper respiratory symptoms due to viral infections, and a potentially safer alternative to prescription drugs for routine cases of the common cold and influenza.
WHAT?!?!
The alternative to antibiotic misuse can only be the correct use of antibiotics. And, in the case of viral infections such as the flu, this can only be the non-use of antibiotics. My trust in this review, published in a SCAM journal of dubious repute, has instantly dropped to zero.
Perhaps a recent overview recently published in THE MEDICAL LETTER provides a more trustworthy picture:
No large randomized, controlled trials evaluating the effectiveness of elderberry for prevention or treatment of influenza have been conducted to date. Elderberry appears to have some activity against influenza virus strains in vitro. In two small studies (conducted outside the US), adults with influenza A or B virus infection taking elderberry extract reported a shorter duration of symptoms compared to those taking placebo. Consuming uncooked blue or black elderberries can cause nausea and vomiting. The rest of the plant (bark, stems, leaves, and root) contains sambunigrin, which can release cyanide. No data are available on the safety of elderberry use during pregnancy or while breastfeeding. CONCLUSION — Prompt treatment with an antiviral drug such as oseltamivir (Tamiflu, and generics) has been shown to be effective in large randomized, controlled trials in reducing the duration of influenza symptoms, and it may reduce the risk of influenza-related complications. There is no acceptable evidence to date that elderberry is effective for prevention or treatment of influenza and its safety is unclear.
Any take-home messages?
Yes:
- Elderberry supplements are not of proven effectiveness against the flu.
- The press officers at universities should be more cautious when writing press-releases.
- They should involve the scientists and avoid the sponsors of the research.
- In-vitro studies can never tell us anything about clinical effectiveness.
- SCAM-journals’ articles must be taken with a pinch of salt.
- Consumers are being misled left, right and centre.
German homeopathy has had a free ride for about 200 years. But, since a few years, critics have started voicing their opposition pointing out that homeopathy lacks evidence of effectiveness. Sales figures, previously in excess of 600 million Euros, have thus started to decline. As a result the homeopathic industry has begun to fight back – as previously discussed, not always by honest or fair means.
Now, a new development has taken place; a manufacturer of homeopathic remedies, Hevert, has sent legal letters to several critics of homeopathy in an attempt to stop them stating that homeopathy is not effective beyond placebo. Failing to abide by this demand would be punishable with a huge find of 5 100 Euros.
After I learnt about this, I had a look on the website of Hevert and found an article detailing the evidence on homeopathy. I think it makes certain things more understandable.
The article concedes that the evidence for homeopathy is often of poor quality and not entirely positive. One problem, the article claims, is the fact that these analyses are usually sponsored by interested parties and that independent, reviews financed through public funds have not been available.
Das Hauptproblem liegt darin, dass die klinische Homöopathieforschung bisher fast ausschließlich über Gelder von komplementärmedizinischen Stiftungen bzw. über die homöopathischen Arzneimittelhersteller selbst finanziert wird. Das heißt, dass eine mit öffentlichen Forschungsgeldern unterstützte systematische Erforschung der klinischen Wirksamkeit von homöopathischen Arzneimitteln bisher nicht stattgefunden hat….
Could it be that Hevert is not well-informed about this point? The fact is that there are now numerous such analyses. Here are the key passages from some of these ‘official verdicts’:
“The principles of homeopathy contradict known chemical, physical and biological laws and persuasive scientific trials proving its effectiveness are not available”
Russian Academy of Sciences, Russia
“Homeopathy should not be used to treat health conditions that are chronic, serious, or could become serious. People who choose homeopathy may put their health at risk if they reject or delay treatments for which there is good evidence for safety and effectiveness.”
National Health and Medical Research Council, Australia
“These products are not supported by scientific evidence.”
Health Canada, Canada
“Homeopathic remedies don’t meet the criteria of evidence-based medicine.”
Hungarian Academy of Sciences, Hungary
“The incorporation of anthroposophical and homeopathic products in the Swedish directive on medicinal products would run counter to several of the fundamental principles regarding medicinal products and evidence-based medicine.”
Swedish Academy of Sciences, Sweden
“There is little evidence to support homeopathy as an effective treatment for any specific condition”
National Centre for Complementary and Integrative Health, USA
“There is no good-quality evidence that homeopathy is effective as a treatment for any health condition”
National Health Service, UK
“Homeopathic remedies perform no better than placebos, and the principles on which homeopathy is based are “scientifically implausible””
House of Commons Science and Technology Committee, UK
“Homeopathy has not definitively proven its efficacy in any specific indication or clinical situation.”
Ministry of Health, Spain
“… homeopathy should be treated as one of the unscientific methods of the so called ‘alternative medicine’, which proposes worthless products without scientifically proven efficacy.”
National Medical Council, Poland
“… there is no valid empirical proof of the efficacy of homeopathy beyond the placebo effect.”
Federaal Kenniscentrum voor de Gezondheidszorg, Belgium
The Hevert article also points out that systematic reviews assessing homeopathy globally are perhaps not ideal, the article claims, and therefore systematic reviews targeted at specific conditions might be preferable and, indeed yield positive findings.
Mittlerweile geht man bei der Auswertung von klinischen Studien sinnvollerweise mehr dazu über, nicht mehr „die Homöopathie“ als Ganzes auf die Probe zu stellen, sondern kleinere Metaanalysen zu festen Indikationen durchzuführen, da dies eindeutigere Ergebnisse liefert….
Again, I suspect that the firm Hevert is not well-informed. I, for instance, have published about a dozen of such reviews which they fail to cite in their article. Let me copy the abstracts of just 4 examples here:
BACKGROUND:
Homeopathy is often advocated for patients with eczema.
OBJECTIVES:
This article systematically reviews the evidence from controlled clinical trials of any type of homeopathic treatment for any type of eczema.
METHODS:
Electronic searches were conducted in Medline, Embase and the Cochrane Library with no restrictions on time or language. In addition, the bibliographies of the retrieved articles and our departmental files were hand searched. All controlled trials of homeopathy in patients with eczema were considered. Their methodological quality was estimated using the Jadad score.
RESULTS:
One randomized and two nonrandomized clinical trials met the inclusion criteria. All were methodologically weak. None demonstrated the efficacy of homeopathy.
CONCLUSIONS:
The evidence from controlled clinical trials therefore fails to show that homeopathy is an efficacious treatment for eczema.
Homoeopathy is often advocated for fibromyalgia (FM) and many FM patients use it. To critically evaluate all randomised clinical trials (RCTs) of homoeopathy as a treatment for FM, six electronic databases were searched to identify all relevant studies. Data extraction and the assessment of the methodological quality of all included studies were done by two independent reviewers. Four RCTs were found, including two feasibility studies. Three studies were placebo-controlled. None of the trials was without serious flaws. Invariably, their results suggested that homoeopathy was better than the control interventions in alleviating the symptoms of FM. Independent replications are missing. Even though all RCTs suggested results that favour homoeopathy, important caveats exist. Therefore, the effectiveness of homoeopathy as a symptomatic treatment for FM remains unproven.
OBJECTIVE:
To assess the evidence of any type of therapeutic or preventive intervention testing homeopathy for childhood and adolescence ailments.
METHODS:
Systematic literature searches were conducted through January 2006 in MEDLINE, EMBASE, AMED, CINAHL, Cochrane Central, British Homeopathic Library, ClinicalTrials.gov, and the UK National Research Register. Bibliographies were checked for further relevant publications. Studies were selected according to predefined inclusion and exclusion criteria. All double-blind, placebo-controlled randomized clinical trials of any homeopathic intervention for preventing or treating childhood and adolescence ailments were included. According to the classification of the World Health Organization, the age range defined for inclusion was 0 to 19 years. Study selection, data extraction, and assessment of methodological quality were performed independently by 2 reviewers.
RESULTS:
A total of 326 articles were identified, 91 of which were retrieved for detailed evaluation. Sixteen trials that assessed 9 different conditions were included in the study. With the exception of attention-deficit/hyperactivity disorder and acute childhood diarrhea (each tested in 3 trials), no condition was assessed in more than 2 double-blind randomized clinical trials. The evidence for attention-deficit/hyperactivity disorder and acute childhood diarrhea is mixed, showing both positive and negative results for their respective main outcome measures. For adenoid vegetation, asthma, and upper respiratory tract infection each, 2 trials are available that suggest no difference compared with placebo. For 4 conditions, only single trials are available.
CONCLUSION:
The evidence from rigorous clinical trials of any type of therapeutic or preventive intervention testing homeopathy for childhood and adolescence ailments is not convincing enough for recommendations in any condition.
Many cancer patients use homeopathic approaches to increase their body’s ability to fight cancer, improve their physical and emotional well-being, and alleviate their pain resulting from the disease or conventional treatments. Homeopathy is highly controversial as there is no plausible mode of action for these highly diluted remedies. The aim of this systematic review is to summarize and critically evaluate the efficacy of homeopathic remedies used as a sole or additional therapy in cancer care. We have searched the literature using the databases: Amed (from 1985); CINHAL (from 1982); EMBASE (from 1974); Medline (from 1951); and CAMbase (from 1998). Randomised and non-randomised controlled clinical trials including patients with cancer or past experience of cancer receiving single or combined homeopathic interventions were included. The methodological quality of the trials was assessed by Jadad score. Six studies met our inclusion criteria (five were randomised clinical trials and one was a non-randomised study); but the methodological quality was variable including some high standard studies. Our analysis of published literature on homeopathy found insufficient evidence to support clinical efficacy of homeopathic therapy in cancer care.
I hasten to add that all of these analyses were funded by public (University) money and not by the industry.
I suspect that Hevert have overlooked all this evidence (no problem, we all can do mistakes!) and therefore, I offer herewith to help them correcting the omissions. In particular, I offer to give an instructive, evidence-based lecture to their staff followed by an in-depth discussion and correction of their website. As homeopathy is under pressure in Germany, I would not even insist on a fee. All I would ask is this: abandon all legal actions against critics of homeopathy; these legal actions are ill-advised and will turn out to be a mere waste of your dwindling profits.
My former institution, the medical school of Vienna, had invited me to give the key-note for a conference entitled ‘Esoterik in der Medizin‘ (22/5/2019). The event was to celebrate the success of a new course for medical students which was initiated after Prof Frass’ lectures on homeopathy had been discontinued. Remarkably, this move had been prompted by complaints from students arguing that Frass was promoting non-evidence-based, bogus concepts.
Whenever I go back to Vienna, I have mixed feelings; pleasant and not so pleasant memories (see below) come to the fore. This time, however, all turned out well, and I was more than delighted.
The new course signifies the realisation that so-called alternative medicine (SCAM) must be covered in any sound medical curriculum. Once graduated, students will be asked by patients about SCAM and have an ethical duty to inform them responsibly. Thus they need to know the essential facts and not the biased perspective that Frass and other enthusiasts tend to convey.
I have always considered this to be important but, as far as I can see, very few medical school manage to deal with this issue adequately. More often than not, the task of running such courses is given to proponents of SCAM who then try to brain-wash the unsuspecting students. The result can be seriously harmful to generations of patients. I am delighted to report that my former medical school has successfully avoided this pitfall. Quackademia has come to an end in Vienna!
In my view, the highlight of the recent event was the students’ presentation of their course-work. They had been supervised in small groups to research selected topics related to SCAM and were given 5 minute slots to present their findings. I truly felt this was impressive. The dedication, the quality of the research and the clarity of the presentations were extraordinary. In my 40 odd years of teaching medical students, I have never seen anything remotely similar (here I should mention perhaps that, 25 years ago when I was teaching in Vienna, medical students seemed to be as unmotivated as they get).
The students’ presentation were followed by 90 minutes of moderated discussion of the audience (the event was open to the public) and 4 experts. Here too, I was positively surprised by the quality of the contributions and the general openness of the debate.
So, overall the both the meeting and, more importantly, the new course for students can be considered a great success, and the organisers must be congratulated on it. For me personally, the most significant aspect was a matter entirely unrelated to SCAM. It was the introductory speech of the dean of the medical school. He announced me as the key-note speaker by praising my research on the Nazi history of the faculty. It was this research that, to some considerable degree, made me leave Vienna in 1993. To see it now appreciated by my former colleagues is deeply moving.
Sophrology is big in France, but almost unknown in English-speaking countries.
What is it?
According to a recent article in ‘The Guardian‘, Sophrology is a system of mind and body, a little bit meditative, a little bit mindful, eastern principles of centredness and focus fed through a European system of rules that can feel just as exotic. It’s been around since the 50s, when a Spanish medical student, Alfonso Caycedo, had the task of administering electroshock treatment to mentally ill patients; sometimes, if that sounds barbaric, inducing insulin comas beforehand. He was an early asker of a question that medicine has confronted more widely since: why does consciousness have to be shaken so violently in order to heal? Implicitly having decided that maybe western medicine may not have all the answers, he concocted this improbable-sounding mix of Tibetan Buddhism, Japanese Zen and yoga, neurology, hypnosis, psychology, psychiatry and relaxation techniques to produce sophrology. It’s huge in Europe – especially in Spain – but it has also been prescribed by Swiss GPs and reportedly used by the French rugby team. It has never cracked the UK.
A bit vague?
Here is a different, perhaps more reliable source.
Sophrology is a non medication-based method which involves both the body and mind. It combines relaxing the muscles, increasing awareness of breathing and positive thinking, and leads to the search for improved well-being through the integration of the body percept. It generates a feeling of “letting go” and helps to relieve physical, psychological and spiritual suffering.
Is there any reliable evidence?
Very little, it seems.
This study (entitled ‘Efficiency of physiotherapy with Caycedian Sophrology on children with asthma: A randomized controlled trial’) aimed to assess whether in children with asthma, peak expiratory flow (PEF) improved more after a sophrology session alongside standard treatment than after standard treatment alone.
The researchers carried out a prospective randomized controlled clinical trial among 74 children aged 6-17 years old, hospitalized for an asthma attack. Group 1: conventional treatment (oxygen, corticosteroids, bronchodilators, physiotherapy) added to one session of sophrology. Group 2: conventional treatment alone. The primary outcome was the PEF variation between the initial and final evaluations (PEF2 -PEF1 ).
Demographic and clinical characteristics were similar in both groups at baseline. Measures before and after the sophrology session showed that the PEF increased by mean 30 L/min in the sophrology group versus 20 L/min in the control group (P = 0.02). Oxygen saturation increased by 1% versus 0% (P = 0.02) and the dyspnea score with visual analogue scale improved by two points point (P = 0.01). No differences were observed between the two groups in terms of duration of hospitalization, use and doses of conventional medical treatment (oxygen, corticosteroids, and bronchodilators), and quality of life scores.
The authors concluded that Sophrology appears as a promising adjuvant therapy to current guideline-based treatment for asthma in children.
The purpose of the only other study was to evaluate the efficiency of sophrology to improve conditions for the realization of non-invasive ventilation (NIV) in patients with acute respiratory failure (ARF). In this prospective randomized and controlled study, consecutive patients with ARF were included. From the very first NIV session, they received either sophrology during the first 30 min of NIV (S group), or standard care by the same nurse during 30 min (T group). The hemodynamic and ventilatory data were recorded continuously; pain, respiratory difficulty and discomfort were measured with a numeric scale at the end of the session.
Thirty patients were included in the study, 27 have been analysed. Each patient received an average of four sessions NIV during the protocol. There was no significant difference between the two groups in terms of improvement in gas exchange. In contrast, there was a significant difference in terms of reduction of difficulty in breathing (-76%), discomfort (-60%) and decrease the pain (-40%) in the sophrology group (p<0.001). Respiratory rate, heart rate and systolic arterial blood pressure were decrease during NIV.
The authors concluded that Sophrology constitutes aid for the achievement of the meetings of NIV in patients’ IRA.
As both studies followed the infamous A+B vs B design, they tell us nothing about the effectiveness of the treatment. This means that sophrology is a therapy that is totally unproven.
Why then is it so popular in France? Search me!
Does its popularity imply that it is effective? No.
The UK Reiki Federation (UKRF) is an independent organisation of individuals who have been attuned to Reiki, with the objective of providing support and guidance to Reiki professionals and to the public, with particular reference to education and training, and the public practice of Reiki. Some of their members give of their time each week to send Reiki healing to anyone who makes a request from anywhere in the world.
Each week the volunteers receive a list of those people/animals/events that have requested healing and they all collectively send positive Reiki healing to everyone on the list.
The UKRF claim that Reiki distant healing (RDH) has now been scientifically proven by Lynne McTaggart in these articles http://www.shareguide.com/McTaggart.html and https://lynnemctaggart.com/the-intention-experiment/ that healing is magnified when many healers are involved, so we are contributing an amazing vibration of positivity into our world and doing so much good, with so little effort. Imagine how brilliant it would be if even more members decided they too wanted to support other people, with minimum effort. It’s so simple just to place your hands on the list and send Reiki to everyone on it. It can be so quick if time is an issue for you and yet so powerful.
A group of UKRF members send Reiki to each other at specific times of the week. They state that we have a list of members’ names and allocated time slots in the week when we can send and receive Reiki energy to each other. The intention is to send Reiki for all the different time slots and then sit down and receive the energy whenever it is convenient for us to do so. Those members who have given me feedback all say they can feel the energy flowing during these times.
I urge you to look up the two ‘scientific proofs’ by McTaggart – I promise, you will not regret the effort. For those who might like to see real evidence for or against RDH, I ran a quick Medline search. Somewhat to my surprise, I did find a rigorous study RDH. Here is its abstract:
In this randomised, double-blinded study, women who underwent an elective C-section were allocated to either usual care (control, n=40) or three distant reiki sessions in addition to usual care (n=40). Pain was assessed using a visual analogue scale (VAS). The primary endpoint was the Area Under the VAS-Time Curve (AUC) for days 1-3. Secondary measures included: the proportion of women who required opioid medications and dose consumed, rate of healing and vital signs.
AUC for pain was not significantly different in the distant reiki and control groups (mean ± SD; 212.1 ± 104.7 vs 223.1 ± 117.8; p=0.96). There were no significant differences in opioid consumption or rate of healing; however, the distant reiki group had a significantly lower heart rate (74.3 ± 8.1 bpm vs 79.8 ± 7.9 bpm, p=0.003) and blood pressure (106.4 ± 9.7 mmHg vs 111.9 ± 11.0 mmHg, p=0.02) post surgery.
CONCLUSION: Distant reiki had no significant effect on pain following an elective C-section.
_______________________________________________________________
This begs at least three questions, in my view:
- Which evidence should I trust, that of McTaggart or that from what seems to be the only RCT on RDH?
- The UK Reiki Foundation state on their website: As the largest Reiki-only professional organisation in the UK and Europe we are setting the highest standard for Reiki. Is the promotion of the McTaggart ‘proof’ combined with the omission from the UKRF site of the only trial of RDH truly in accordance with the highest standards?
- Is a professional organisation that does such things really professional?


