clinical trial
Reflexology is an alternative therapy that is subjectively pleasant and objectively popular; it has been the subject on this blog before (see also here and here). Reflexologists assume that certain zones on the sole of our feet correspond to certain organs, and that their manual treatment can influence the function of these organs. Thus reflexology is advocated for all sorts of conditions, including infant colic.
The aim of this new study was to explore the effect of reflexology on infantile colic.
A total of 64 babies with colic were included in this study. Following a paediatrician’s diagnosis, two groups (study and control) were created. Socio-demographic data (including mother’s age, educational status, and smoking habits of parents) and medical history of the baby (including gender, birth weight, mode of delivery, time of the onset breastfeeding after birth, and nutrition style) were collected. The Infant Colic Scale (ICS) was used to estimate the colic severity in the infants. Reflexology was applied to the study group by the researcher and their mother 2 days a week for 3 weeks. The babies in the control group did not receive reflexology. Assessments were performed before and after the intervention in both groups.
The results show that the two groups were similar regarding socio-demographic background and medical history. While there was no difference between the groups in ICS scores before application of reflexology, the mean ICS score of the study group was significantly lower than that of control group at the end of the intervention.
The authors concluded that reflexology application for babies suffering from infantile colic may be a promising method to alleviate colic severity.
The authors seem to attribute the outcome to specific effects of reflexology.
However, they are mistaken!
Why?
Because their study does not control for the non-specific effects of the intervention.
Reflexology has not been shown to work for anything (“the best clinical evidence does not demonstrate convincingly reflexology to be an effective treatment for any medical condition“), and there is plenty of evidence to show that holding the baby, massaging it, cuddling it, rocking it or doing just about anything with it will have an effect, e. g.:
…kangaroo care for infants with colic is a promising intervention…
I think, in a way, this is rather good news; we do not need to believe in the hocus-pocus of reflexology in order to help our crying infants.
I hear this argument so regularly that it might be worth analysing it (yet again) a bit closer.
It is used with the deepest of convictions by proponents of all sorts of quackery who point out that science does not know or explain everything – and certainly not their (very special) therapy. Science is just not sophisticated enough, they say; in fact, a few years ago, it could not even explain how Aspirin works. And just like Aspirin, their very special therapy – let’s call it energy healing (EH) for the sake of this post – does definitely and evidently work. There even is ample proof:
- Patients get better after using EH, and surely patients don’t lie.
- Patients pay for EH, and who would pay for something that does not work?
- EH has survived hundreds of years, and ineffective therapies don’t.
- EH practitioners have tons of experience and therefore know best.
- They are respected by very important people and organisations.
- EH is even reimbursed by some insurance companies.
You have all heard the argument, I’m sure.
How to respond?
The ‘proofs’ listed above are simply fallacies; as such they do not need more detailed discussions, I hope.
But how can we refute the notion that science is not yet sufficiently advanced to explain EH?
The simplest approach might be to explain that science has already tested EH and found it to be ineffective. There really is nothing more to say. And the often-quoted example of Aspirin does clearly not wash. True, a few decades ago, we did not know how it worked. But we always knew that it worked because we conducted clinical trials, and they generated positive results. These findings we the main reasons why scientists wanted to find out how it works, and eventually they did (and even got a Nobel Prize for it). Had the clinical trials not shown effectiveness, nobody would have been interested in alleged mechanisms of action.
With EH, things are different. Rigorous clinical trials of EH have been conducted, and the totality of this evidence fails to show that EH works. Therefore, chasing after a mechanism of action would be silly and wasteful. It’s true, science cannot explain EH, but this is not because it is not yet sophisticated enough; it is because there is nothing to explain. EH has been disproven, and waffling about ‘science is not yet able to explain it’ is either a deliberate lie or a serious delusion.
So far so good. But what if EH had not been submitted to clinical trials?
In such cases, the above line of argument would not work very well.
For instance, as far as I know, there is not a single rigorous clinical trial of crystal healing (CH). Does that mean that perhaps CH-proponents are correct when claiming that it does evidently work and science simply cannot yet understand how?
No, I don’t think so.
Like most of the untested alternative therapies, CH is not based on plausible assumptions. In fact, the implausibility of the underlying assumptions is the reason why such treatments have not and probably never will be submitted to rigorous clinical trials. Why should anyone waste his time and our money running expensive tests on something that is so extremely unlikely? Arguably doing so would even be unethical.
With highly implausible therapies we need no trials, and we do not need to fear that science is not yet sufficiently advance to explain them. In fact, science is sufficiently advanced to be certain that there can be no explanation that is in line with the known laws of nature.
Sadly, some truly deluded fans of CH might still not be satisfied and respond to our reasoning that we need a ‘paradigm shift’. They might say that science cannot explain CH because it is stuck in the straightjacket of an obsolete paradigm which does not cater for phenomena like CH.
Yet this last and desperate attempt of the fanatics is not a logical refuge. Paradigm shifts are not required because some quack thinks so, they are needed only if data have been emerging that cannot possibly be explained within the current paradigm. But this is never the case in alternative medicine. We can explain all the experience of advocates, positive results of researchers and ‘miracle’ cures of patients that are being reported. We know that the experiences are real, but are sure that their explanations of the experience are false. They are not due to the treatment per se but to other phenomena such as placebo effects, natural history, regression towards the mean, spontaneous recovery, etc.
So, whichever way we turn things, and whichever way enthusiasts of alternative therapies twist them, their argument that ‘SCIENCE IS NOT YET ABLE TO EXPLAIN’ is simply wrong.
Since many months, I have noticed a proliferation of so-called pilot studies of alternative therapies. A pilot study (also called feasibility study) is defined as a small scale preliminary study conducted in order to evaluate feasibility, time, cost, adverse events, and improve upon the study design prior to performance of a full-scale research project. Here I submit that most of the pilot studies of alternative therapies are, in fact, bogus.
To qualify as a pilot study, an investigation needs to have an aim that is in line with the above-mentioned definition. Another obvious hallmark must be that its conclusions are in line with this aim. We do not need to conduct much research to find that even these two elementary preconditions are not fulfilled by the plethora of pilot studies that are currently being published, and that proper pilot studies of alternative medicine are very rare.
Three recent examples of dodgy pilot studies will have to suffice (but rest assured, there are many, many more).
The aim of this study was to evaluate the effects of foot reflexotherapy on pain and postural balance in elderly individuals with low back pain. And the conclusions drawn by its authors were that this study demonstrated that foot reflexotherapy induced analgesia but did not affect postural balance in elderly individuals with low back pain.
The aim of this study was to investigate the effect of Tai Chi training on dual-tasking performance that involved stepping down and compared it with that of conventional exercise among stroke survivors. And the conclusions read: These results suggest a beneficial effect of Tai Chi training on cognition among stroke survivors without compromising physical task performance in dual-tasking.
The aim of this study was to evaluate the efficacy [of acupuncture] over 12 weeks of treatment and 12 weeks of follow-up. And the conclusion: Acupuncture decreases WC, HC, HbA1c, TG, and TC values and blood pressure in MetS.
It is almost painfully obvious that these studies are not ‘pilot’ studies as defined above.
So, what are they, and why are they so popular in alternative medicine?
The way I see it, they are the result of amateur researchers conducting pseudo-research for publication in lamentable journals in an attempt to promote their pet therapies (I have yet to find such a study that reports a negative finding). The sequence of events that lead to the publication of such pilot studies is usually as follows:
- An enthusiast or a team of enthusiasts of alternative medicine decide that they will do some research.
- They have no or very little know-how in conducting a clinical trial.
- They nevertheless feel that such a study would be nice as it promotes both their careers and their pet therapy.
- They design some sort of a plan and start recruiting patients for their trial.
- At this point they notice that things are not as easy as they had imagined.
- They have too few funds and too little time to do anything properly.
- This does, however, not stop them to continue.
- The trial progresses slowly, and patient numbers remain low.
- After a while the would-be researchers get fed up and decide that their study has enough patients to stop the trial.
- They improvise some statistical analyses with their results.
- They write up the results the best they can.
- They submit it for publication in a 3rd class journal and, in order to get it accepted, they call it a ‘pilot study’.
- They feel that this title is an excuse for even the most obvious flaws in their work.
- The journal’s reviewers and editors are all proponents of alternative medicine who welcome any study that seems to confirm their belief.
- Thus the study does get published despite the fact that it is worthless.
Some might say ‘so what? no harm done!’
But I beg to differ: these studies pollute the medical literature and misguide people who are unable or unwilling to look behind the smoke-screen. Enthusiasts of alternative medicine popularise these bogus trials, while hiding the fact that their results are unreliable. Journalists report about them, and many consumers assume they are being told the truth – after all it was published in a ‘peer-reviewed’ medical journal!
My conclusions are as simple as they are severe:
- Such pilot studies are the result of gross incompetence on many levels (researchers, funders, ethics committees, reviewers, journal editors).
- They can cause considerable harm, because they mislead many people.
- In more than one way, they represent a violation of medical ethics.
- The could be considered scientific misconduct.
- We should think of stopping this increasingly common form of scientific misconduct.
Reiki is a Japanese technique administered by “laying on hands” and is based on the idea that an unseen “life force energy” flows through us and is what causes us to be alive. If one’s “life force energy” is low, then we are more likely to get sick or feel stress, and if it is high, we are more capable of being happy and healthy (because it is such a clear-cut case of nonsense, we have discussed Reiki regularly; see for instance here, here, here, here, here, here, and here).
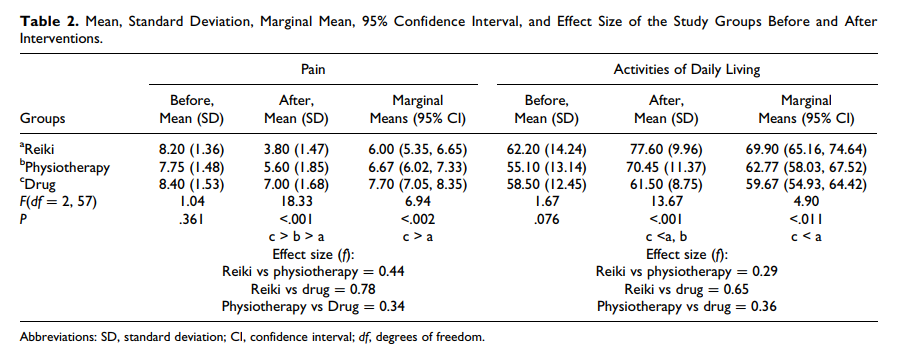
But nonsense does not stop researchers from conducting trials. In this new clinical trial, conducted in Physiotherapy Clinic of Khatam Al-Anbia Hospital in Iran, 60 patients with pain due to inter-vertebral disc herniation (IVDH) were randomly assigned to one of three groups.
- The Reiki group received three 15-minute Reiki sessions on consecutive days by a master of Reiki plus Indomethacin and Methocarbamol (as in group 3).
- The physiotherapy group underwent 7 to 10 sessions of physiotherapy of 60 to 90 minutes (heat therapy, TENS, pelvic traction, and physical exercises) plus Indomethacin and Methocarbamol (as in group 3).
- The drug group received Indomethacin capsules 75 mg and Methocarbamol tablets 500 mg every 8 hours daily for one week.
The severity of pain and the activities of daily living (ADL) were measured using visual analogue scales (VAS) and ADL-Instrumental ADL questionnaire before and after the intervention. A significant difference was found in pain intensity and ADL improvement between Reiki and the drug therapy. No significant difference between the Reiki and physiotherapy groups were noted.
The authors concluded that Reiki and physiotherapy are effective methods in managing pain and improving ADL in patients with IVDH; however, Reiki is more cost-effective and faster treatment method than physiotherapy.
This RCT seems fairly well-panned and conducted, and its results are straight forward. My only problem with it is how the findings are interpreted.
The study design was such that there was no blinding or control for placebo effects. Therefore, the observed outcomes can be interpreted in more than one way. In my view, by far the most plausible explanation is that Reiki (being an exotic, impressive intervention that generates plenty of expectation) produced a powerful placebo effect. Physiotherapy (being entirely normal and routine), on the other hand, was only marginally successful. It is regrettable that the authors do not even consider this interpretation of their results. They should have remembered that a clinical trial test the null-hypothesis (the experimental treatment is not better that the comparator) which can be rejected only, if there is no other reasonable explanation for the results produced.
If I am correct, the conclusions should be re-written as follows:
The addition of Reiki to drug treatment generated better outcomes than drug therapy alone. Physiotherapy was only marginally effective. The effects of Reiki are most likely not due to the treatment per se but to a classical placebo response.
Homeopathy works!
At least this is what the authors of this new study want us to believe.
But are they right?
This RCT is entitled ‘Efficacy and tolerability of a complex homeopathic drug in children suffering from dry cough-A double-blind, placebo- controlled, clinical trial’. It recruited children suffering from acute dry cough to assess the efficacy and tolerability of a complex homeopathic remedy in liquid form (Drosera, Coccus cacti, Cuprum Sulfuricum, Ipecacuanha = Monapax syrup, short: verum).
The authors stated that “preparations of Drosera, Coccus cacti, Cuprum sulfuricum, and Ipecacuanha are well-known antitussives in homeopathic medicine. Each of them is connected with special subtypes of cough. Drosera is intended for inflammations of the respiratory tract, especially for whooping cough. Coccus cacti is intended for inflammations of the nasopharyngeal space and the respiratory tract. Cuprum sulfuricum is intended for spasmodic coughing at night. Ipecacuanha is intended for bronchitis, bronchial asthma, and whooping cough. The complex homeopathic drug explored in this trial consists of all four of these active substances.”
According to the authors of the paper, “the primary objective of the trial was to demonstrate the superiority of verum compared to the placebo”.
A total of 89 children, enrolled in the Ukraine between 15/04/2008 and 26/05/2008 in 9 trial centres, received verum and 91 received placebo daily for 7 days (age groups 0.5–3, 4–7 and 8–12 years). The trial was conducted using an adaptive 3-stage group sequential design with possible sample size adjustments after the two planned interim analyses. The inverse normal method of combining the p-values from all three stages was used for confirmatory hypothesis testing at the interim analyses as well as at the final analysis. The primary efficacy variable was the improvement of the Cough Assessment Score. Tolerability and compliance were also assessed. A confirmatory statistical analysis was performed for the primary efficacy variable and a descriptive analysis for the secondary parameters.
A total of 180 patients (89 in the verum and 91 in the placebo group) evaluable according to the intention-to-treat principle were included in the trial. The Cough Assessment Score showed an improvement of 5.2 ± 2.6 points for children treated with verum and 3.2 ± 2.6 points in the placebo group (p < 0.0001). The difference of the least square means of the improvements was 1.9 ± 0.4. The effect size of Cohen´s d was d = 0.77. In all secondary parameters the patients in the verum group showed higher rates of improvement and remission than those in the placebo group. In 15 patients (verum: n = 6; placebo: n = 9) 18 adverse drug reactions of mild or moderate intensity were observed.
The authors concluded that the administering verum resulted in a statistically significantly greater improvement of the Cough Assessment Score than the placebo. The tolerability was good and not inferior to that of the placebo.
This study seems fairly rigorous. What is more, it has been published in a mainstream journal of reasonably high standing. So, how can its results be positive? We all know that homeopathy does not work, don’t we?
Are we perhaps mistaken?
Are highly diluted homeopathic remedies effective after all?
I don’t think so.
Let me explain to you a few points that raise my suspicions about this study:
- It was conducted 10 years ago; why did it take that long to get it published?
- I don’t think highly of a study with “the primary objective … to demonstrate the superiority” of the experimental interventions. Scientists use RCTs for testing efficacy and pseudo-scientist use it for demonstrating it, I think.
- The study was conducted in the Ukraine in 9 centres, yet no Ukrainian is an author of the paper, and there is not even an acknowledgement of these primary investigators.
- The ‘adaptive 3-stage group sequential design with possible sample size adjustments’ sounds very odd to me, but I may be wrong; I am not a statistician.
- We learn that 180 patients were evaluated, but not how many were entered into the trial?
- The Cough Assessment Score is not a validated outcome measure.
- Was the verum distinguishable from the placebo? It would be easy to test whether the patients/parents were truly blinded. Yet no such results were included.
- The trial was funded by the manufacturer of the homeopathic remedy.
- The paper has three authors 1)Hans W. Voß has no conflict of interest to declare. 2) Rainer Brünjes is employed at Cassella-med, the marketing authorisation holder of the study product. 3) Andreas Michalsen has consulted for Cassella-med and participated in advisory boards.
I know, homeopathy fans will think I am nit-picking; and perhaps they are correct. So, let me tell you why I really do strongly reject the notion that this study shows or even suggests that highly diluted homeopathic remedies are more than placebos.
The remedy used in this study is composed of Drosera 0,02 g, Hedera helix Ø 0,04 g, China D1 0,02 g, Coccus cacti D1 0,04 g, Cuprum sulfuricum D4 2,0 g, Ipecacuanha D4 2,0 g, Hyoscyamus D4 2,0 g.
In case you don’t know what ‘Ø’ stands for (I don’t blame you, hardly anyone outside the world of homeopathy does), it signifies a ‘mother tincture’, i. e. an undiluted herbal extract; and ‘D1’ signifies diluted 1:10. This means that the remedy may be homeopathic from a regulatory point of view, but for all intents and purposes it is a herbal medicine. It contains an uncounted amount of active compounds, and it is therefore hardly surprising that it might have pharmacological effects. In turn, this means that this trial does by no means overturn the fact that highly diluted homeopathic remedies are pure placebos.
It’s a pity, I find, that the authors of the paper fail to explain this simple fact in full detail – might one think that they intentionally aimed at misleading us?
As I often said, I find it regrettable that sceptics often say THERE IS NOT A SINGLE STUDY THAT SHOWS HOMEOPATHY TO BE EFFECTIVE (or something to that extent). This is quite simply not true, and it gives homeopathy-fans the occasion to suggest sceptics wrong. The truth is that THE TOTALITY OF THE MOST RELIABLE EVIDENCE FAILS TO SUGGEST THAT HIGHLY DILUTED HOMEOPATHIC REMEDIES ARE EFFECTIVE BEYOND PLACEBO. As a message for consumers, this is a little more complex, but I believe that it’s worth being well-informed and truthful.
And that also means admitting that a few apparently rigorous trials of homeopathy exist and some of them show positive results. Today, I want to focus on this small set of studies.
How can a rigorous trial of a highly diluted homeopathic remedy yield a positive result? As far as I can see, there are several possibilities:
- Homeopathy does work after all, and we have not fully understood the laws of physics, chemistry etc. Homeopaths favour this option, of course, but I find it extremely unlikely, and most rational thinkers would discard this possibility outright. It is not that we don’t quite understand homeopathy’s mechanism; the fact is that we understand that there cannot be a mechanism that is in line with the laws of nature.
- The trial in question is the victim of some undetected error.
- The result has come about by chance. Of 100 trials, 5 would produce a positive result at the 5% probability level purely by chance.
- The researchers have cheated.
When we critically assess any given trial, we attempt, in a way, to determine which of the 4 solutions apply. But unfortunately we always have to contend with what the authors of the trial tell us. Publications never provide all the details we need for this purpose, and we are often left speculating which of the explanations might apply. Whatever it is, we assume the result is false-positive.
Naturally, this assumption is hard to accept for homeopaths; they merely conclude that we are biased against homeopathy and conclude that, however, rigorous a study of homeopathy is, sceptics will not accept its result, if it turns out to be positive.
But there might be a way to settle the argument and get some more objective verdict, I think. We only need to remind ourselves of a crucially important principle in all science: INDEPENDENT REPLICATION. To be convincing, a scientific paper needs to provide evidence that the results are reproducible. In medicine, it unquestionably is wise to accept a new finding only after it has been confirmed by other, independent researchers. Only if we have at least one (better several) independent replications, can we be reasonably sure that the result in question is true and not false-positive due to bias, chance, error or fraud.
And this is, I believe, the extremely odd phenomenon about the ‘positive’ and apparently rigorous studies of homeopathic remedies. Let’s look at the recent meta-analysis of Mathie et al. The authors found several studies that were both positive and fairly rigorous. These trials differ in many respects (e. g. remedies used, conditions treated) but they have, as far as I can see, one important feature in common: THEY HAVE NOT BEEN INDEPENDENTLY REPLICATED.
If that is not astounding, I don’t know what is!
Think of it: faced with a finding that flies in the face of science and would, if true, revolutionise much of medicine, scientists should jump with excitement. Yet, in reality, nobody seems to take the trouble to check whether it is the truth or an error.
To explain this absurdity more fully, let’s take just one of these trials as an example, one related to a common and serious condition: COPD
The study is by Prof Frass and was published in 2005 – surely long enough ago for plenty of independent replications to emerge. Its results showed that potentized (C30) potassium dichromate decreases the amount of tracheal secretions was reduced, extubation could be performed significantly earlier, and the length of stay was significantly shorter. This is a scientific as well as clinical sensation, if there ever was one!
The RCT was published in one of the leading journals on this subject (Chest) which is read by most specialists in the field, and it was at the time widely reported. Even today, there is hardly an interview with Prof Frass in which he does not boast about this trial with truly sensational results (only last week, I saw one). If Frass is correct, his findings would revolutionise the lives of thousands of seriously suffering patients at the very brink of death. In other words, it is inconceivable that Frass’ result has not been replicated!
But it hasn’t; at least there is nothing in Medline.
Why not? A risk-free, cheap, universally available and easy to administer treatment for such a severe, life-threatening condition would normally be picked up instantly. There should not be one, but dozens of independent replications by now. There should be several RCTs testing Frass’ therapy and at least one systematic review of these studies telling us clearly what is what.
But instead there is a deafening silence.
Why?
For heaven sakes, why?
The only logical explanation is that many centres around the world did try Frass’ therapy. Most likely they found it does not work and soon dismissed it. Others might even have gone to the trouble of conducting a formal study of Frass’ ‘sensational’ therapy and found it to be ineffective. Subsequently they felt too silly to submit it for publication – who would not laugh at them, if they said they trailed a remedy that was diluted 1: 1000000000000000000000000000000000000000000000000000000000000 and found it to be worthless? Others might have written up their study and submitted it for publication, but got rejected by all reputable journals in the field because the editors felt that comparing one placebo to another placebo is not real science.
And this is roughly, how it went with the other ‘positive’ and seemingly rigorous studies of homeopathy as well, I suspect.
Regardless of whether I am correct or not, the fact is that there are no independent replications (if readers know any, please let me know).
Once a sufficiently long period of time has lapsed and no replications of a ‘sensational’ finding did not emerge, the finding becomes unbelievable or bogus – no rational thinker can possibly believe such a results (I for one have not yet met an intensive care specialist who believes Frass’ findings, for instance). Subsequently, it is quietly dropped into the waste-basket of science where it no longer obstructs progress.
The absence of independent replications is therefore a most useful mechanism by which science rids itself of falsehoods.
It seems that homeopathy is such a falsehood.
We have repeatedly discussed the journal ‘Evidence-Based Complementary and Alternative Medicine’ (see for instance here and here). The journal has recently done something remarkable and seemingly laudable: it retracted an article titled “Psorinum Therapy in Treating Stomach, Gall Bladder, Pancreatic, and Liver Cancers: A Prospective Clinical Study” due to concerns about the ethics, authorship, quality of reporting, and misleading conclusions.***
Aradeep and Ashim Chatterjee own and manage the Critical Cancer Management Research Centre and Clinic (CCMRCC), the private clinic to which they are affiliated. The methods state “The study protocol was approved by the Institutional Review Board (IRB approval Number: 2001–05) of the CCMRCC” in 2001, but a 2014 review of Psorinum therapy said CCMRCC was founded in 2008. The study states “The participants received the drug Psorinum along with allopathic and homeopathic supportive treatments without trying conventional or any other investigational cancer treatments”; withholding conventional cancer treatment raises ethical concerns.
We asked the authors and their institutions for documentation of the ethics approval, the study protocol, and a blank copy of the informed consent form. However, the corresponding author, Aradeep Chatterjee, was reported to have been arrested in June 2017 for allegedly practising medicine without the correct qualifications and his co-author and father Ashim Chatterjee was reported to have been arrested in August; the Chatterjees and their legal representative did not respond to our queries. The co-authors Syamsundar Mandal, Sudin Bhattacharya, and Bishnu Mukhopadhyay said they did not agree to be authors of the article and were not aware of its submission; co-author Jaydip Biswas did not respond.
A member of the editorial board noted that although the discussion stated that “The limitation of this study is that it did not have any placebo or treatment control arm; therefore, it cannot be concluded that Psorinum Therapy is effective in improving the survival and the quality of life of the participants due to the academic rigours of the scientific clinical trials”, the abstract was misleading because it implied Psorinum therapy is effective in cancer treatment. The study design was described as a “prospective observational clinical trial”, but it cannot have been both observational and a clinical trial.
(*** while I wrote this blog (13/3/18) the abstract of this paper was still available on Medline without a retraction notice)
________________________________________________________________________________
In case you wonder what ‘psorinum therapy’ is, this website explains:
A cancer specialist and Psorinum clinical researcher, Aurodeep Chaterjee, believes Psorinum Therapy is less time consuming and more economical for treatment of cancer. ‘The advantage of this treatment is that the patient can continue this treatment while staying home and the hospitalization is less required,’ said Chaterjee. He added that it’s an immunotherapy treatment in which the medicine is in liquid form and the technique of consumption is oral.
Though no chemo or radiation sessions are required in it but they can be used parallel to it depending upon the stage of the cancer. He claimed that more than 30 types of cancers could be treated from this therapy. Some of them include gastrointestinal cancer, liver cancer, gall bladder cancer, ovarian cancer, stomach cancer, etc. The process requires two months duration in which the patient has to undergo 12 cycles and the cost is just Rs 5000. Moli Rapoor 55, software engineer from USA who is suffering from ovarian cancer said on Thursday (June 20) that after three chemo cycles when her cancer did not cure after being diagnosed in 2008, she decided to take up Psorinum therapy.
_________________________________________________________________________________
I am sorry, but the retraction of such a paper is far less laudable than it seems – it should not have been retracted, but it should have never been published in the first place. There are multiple points where the reviewers’ and editors’ alarm bells should have started ringing loud and clear. Take, for instance, this note at the end of the paper:
Funding
Dr. Rabindranath Chatterjee Memorial Cancer Trust provided funding for this study.
Conflict of Interests
The authors declare that they have no conflict of interests.
I think that this should have been a give-away, considering the names of the authors: Chatterjee A1, Biswas J, Chatterjee A, Bhattacharya S, Mukhopadhyay B, Mandal S.
What this story shows, in my view, is that the journal ‘Evidence-Based Complementary and Alternative Medicine’ (EBCAM) operates an unacceptably poor system of peer-review, and is led by an editor who seems to shut both eyes when deciding about publication or rejection. And why would an editor shut his/her eyes to abuse? Perhaps the journal’s interesting business model provides an explanation? Here is what I wrote about it previously:
What I fail to understand is why so many researchers send their papers to this journal. In 2015, EBCAM published just under 1000 (983 to be exact) papers. This is not far from half of all Medline-listed articles on alternative medicine (2056 in total).
To appreciate these figures – and this is where it gets not just puzzling but intriguing, in my view – we need to know that EBCAM charges a publication fee of US$ 2500. That means the journal has an income of about US$ 2 500 000 per annum!
END OF QUOTE
To put it in a nutshell: in healthcare, fraud and greed can cause enormous harm.
The UK ACUPPUNCTURE RESEARCH RESOURCE CENTRE (ARRC) is a specialist resource for acupuncture research information; the only such resource in the land. It is funded by the British Acupuncture Council (BAcC) and was established in 1994 by the BAcC in partnership with the Foundation for Research in Traditional Chinese Medicine.
The ARRC organise an annual meeting. This year’s meeting is special because it is their 20th! It is scheduled to take place in London on 17th March. In case you are already busy that day, or you want to save the £120 registration fee, I have copied for you the programme below and am even able to inform you about the content of each lecture.
- Hugh MacPherson – Celebrating twenty years of acupuncture research
- Lee Hullender Rubin – The Impact of Whole Systems Traditional Chinese Medicine on In Vitro Fertilization Outcomes – A Retrospective Cohort Study
- Robert Davis – Beyond Efficacy: Conducting and translating research for policy-makers considering acupuncture reimbursement in a small, rural US state
- Lee Hullender Rubin – Acupuncture Augmentation of Lidocaine Treatment of Provoked, localized Vulvodynia – a Feasability and Acceptability Pilot Study
- Florian Beissner – A TCM-based psychotherapy with acupuncture for endometriosis
- Beverley De Valois – Using moxa on St 36 to reduce chemotherapy-induced pancytopenia: a feasibility study
- Ian Appleyard – Warm needle acupuncture for osteoarthritis of the knee: a pilot study
- Ed Fraser – Stand Easy: An Evaluation of the acceptability and effectiveness of acupuncture as a treatment for post-traumatic stress disorder for veterans in Norfolk
Having attended plenty of such meetings in my time, I can give you a fairly good idea about the contents of the 8 lectures. Below, I provide succinct (and slightly satirical) summaries of what the presenters will tell their audience on the 17th:
- Despite difficult circumstances, we (the ARRC) have done very well indeed. We managed to publish lots of papers, and we made sure that not a single one reported a negative result. That would be bad for business. We are optimistic about the future provided we get some funding, of course.
- Whole Systems Traditional Chinese Medicine has a profoundly positive effect on the outcomes of In Vitro fertilization. We are totally balled over! Only the most pedantic sceptics would have reservations and might argue that the study had no controls and was retrospective. But who cares, we believe in positive results, and therefore, we never listen to criticism.
- Because efficacy is a sticky issue in the realm of acupuncture, it is much wiser to tackle policy makers by persuading them that they can save money (lots of it), if they implement the abundant use of acupuncture. The evidence for this notion is flimsy to say the least, but policy makers do not understand the science (and neither do we).
- Our study showed that Acupuncture Augmentation of Lidocaine Treatment is extremely good for vulvodynia. We are very impressed, over the moon even. Of course, this was a feasibility study and we should really only conclude that a full study may be feasible, but let’s not be nit-picking.
- Based on my very extensive experience, I am able to confirm that TCM-based psychotherapy with acupuncture is an excellent therapy for endometriosis. Rigorous, controlled clinical trials do not exist, but my findings are so clear that, quite honestly, we do not need them.
- Using moxa on St 36 to reduce chemotherapy-induced pancytopenia is feasible. Isn’t that lovely?
- My trial of warm needle acupuncture for osteoarthritis of the knee showed most encouraging results. Of course, this was only a pilot study, and from it we should really only conclude that a proper study may be feasible, but let’s not be holier than thou!
- Our results demonstrate that acupuncture as a treatment for post-traumatic stress disorder is amazingly effective. A breakthrough! What is more, veterans found it most acceptable. The study is not rigorous, but I don’t mind. I advocate this treatment to be rolled out nationally as a matter of urgency.
————————————————————————————————————————————
So, there you are; that’s all you need to know about the 20th annual meeting of the ARRC.
You don’t need to go.
I have thus saved you £120!
No, I don’t expect thanks – I prefer, if you would send half of this amount (£60) to my account.
Clinical trials are a most useful tool, but they can easily be abused. It is not difficult to misuse them in such a way that even the most useless treatment appears to be effective. Sadly, this sort of thing happens all too often in the realm of alternative medicine. Take for instance this recently published trial of homeopathy.
The objective of this study was to investigate the usefulness of classical homeopathy for the prevention of recurrent urinary tract infections (UTI) in patients with spinal cord injury (SCI). Patients were admitted to this trial, if they had chronic SCI and had previously suffered from at least three UTI/year. They were treated either with a standardized prophylaxis alone, or with a standardized prophylaxis in combination with homeopathy. The number of UTIs, general and specific quality of life (QoL), and satisfaction with homeopathic treatment were assessed prospectively over the period of one year. Ten patients were in the control group and 25 patients received adjunctive homeopathic treatment. The median number of self-reported UTI in the homeopathy group decreased significantly, whereas it remained unchanged in the control group. The domain incontinence impact of the KHQ improved significantly, whereas the general QoL did not change. The satisfaction with homeopathic care was high.
The authors concluded that adjunctive homeopathic treatment lead to a significant decrease of UTI in SCI patients. Therefore, classical homeopathy could be considered in SCI patients with recurrent UTI.
Where to begin?
Here are just some of the most obvious flaws of and concerns with this study:
- There is no plausible rationale to even plan such a study.
- The sample size was far too small for allowing generalizable conclusions.
- There was no adequate randomisation and patients were able to chose the homeopathy option.
- The study seems to lack objective outcome measures.
- The study design did not allow to control for non-specific effects; therefore, it seems likely that the observed outcomes are unrelated to the homeopathic treatments but are caused by placebo and other non-specific effects.
- Even if the study had been rigorous, we would need independent replications before we draw such definitive conclusions.
- Two of the authors are homeopaths, and it is in their clinics that the study took place.
- Some of the authors have previously published a very similar paper – except that this ‘case series’ included no control group at all.
- The latter paper seems to have been published more than once.
- Of this paper, one of the authors claimed that ” the usefulness of classical homeopathy as an adjunctive measure for UTI prophylaxis in patients with NLUTD due to SCI has been demonstrated in a case series”. He seems to be unaware of the fact that a case series cannot possible lend itself to demonstrate this.
- I do wonder: did they just add a control group to their case series thus pretending it became a controlled clinical trial?
What strikes me most with such pseudo-research is its abundance and the naivety – or should I call it ignorance? – of the enthusiasts who conduct it. Most of them, I am fairly sure do not mean to do harm; but by Jove they do!
The question whether spinal manipulative therapy (SMT) has any specific therapeutic effects is still open. This fact must irritate ardent chiropractors, and they therefore try everything to dispel our doubts. One way would be to demonstrate a dose-effect relationship between SMT and the clinical outcome. But, for several reasons, this is not an easy task.
This RCT was aimed at identifying the dose-response relationship between visits for SMT and chronic cervicogenic headache (CGH) outcomes; to evaluate the efficacy of SMT by comparison with a light massage control.
The study included 256 adults with chronic CGH. The primary outcome was days with CGH in the prior 4 weeks evaluated at the 12- and 24-week primary endpoints. Secondary outcomes included CGH days at remaining endpoints, pain intensity, disability, perceived improvement, medication use, and patient satisfaction. Participants were randomized to 4 different dose levels of chiropractic SMT: 0, 6, 12, or 18 sessions. They were treated 3 times per week for 6 weeks and received a focused light-massage control at sessions when SMT was not assigned. Linear dose effects and comparisons to the no-manipulation control group were evaluated at 6, 12, 24, 39, and 52 weeks.
A linear dose-response was observed for all follow-ups, a reduction of approximately 1 CGH day/4 weeks per additional 6 SMT visits (p<.05); a maximal effective dose could not be determined. CGH days/4 weeks were reduced from about 16 to 8 for the highest and most effective dose of 18 SMT visits. Mean differences in CGH days/4 weeks between 18 SMT visits and control were -3.3 (p=.004) and -2.9 (p=.017) at the primary endpoints, and similar in magnitude at the remaining endpoints (p<.05). Differences between other SMT doses and control were smaller in magnitude (p > .05). CGH intensity showed no important improvement nor differed by dose. Other secondary outcomes were generally supportive of the primary.
The authors concluded that there was a linear dose-response relationship between SMT visits and days with CGH. For the highest and most effective dose of 18 SMT visits, CGH days were reduced by half, and about 3 more days per month than for the light-massage control.
This trial would make sense, if the effectiveness of SMT for CGH had been a well-documented fact, and if the study had rigorously controlled for placebo-effects.
But guess what?
Neither of these conditions were met.
A recent review concluded that there are few published randomized controlled trials analyzing the effectiveness of spinal manipulation and/or mobilization for TTH, CeH, and M in the last decade. In addition, the methodological quality of these papers is typically low. Clearly, there is a need for high-quality randomized controlled trials assessing the effectiveness of these interventions in these headache disorders. And this is by no means the only article making such statements; similar reviews arrive at similar conclusions. In turn, this means that the effects observed after SMT are not necessarily specific effects due to SMT but could easily be due to placebo or other non-specific effects. In order to avoid confusion, one would need a credible placebo – one that closely mimics SMT – and make sure that patients were ‘blinded’. But ‘light massage’ clearly does not mimic SMT, and patients obviously were aware of which interventions they received.
So, an alternative – and I think at least as plausible – conclusion of the data provided by this new RCT is this:
Chiropractic SMT is associated with a powerful placebo response which, of course, obeys a dose-effect relationship. Thus these findings are in keeping with the notion that SMT is a placebo.
And why would the researchers – who stress that they have no conflicts of interest – mislead us by making this alternative interpretation of their findings not abundantly clear?
I fear, the reason might be simple: they also seem to mislead us about their conflicts of interest: they are mostly chiropractors with a long track record of publishing promotional papers masquerading as research. What, I ask myself, could be a stronger conflict of interest?
(Pity that a high-impact journal like SPINE did not spot these [not so little] flaws)