antioxidants
A journalist from the DAILY MAIL alerted me to the fact that yet another celebrity having decided to sell dietary supplements, interviewed me on the subject, and eventually published an article about it. One would not have thought that the Beckhams are short of money – so, why did David Beckham turn into a snake-oil salesman? I am far from being able to answer this question. What I now do know is that, via his firm ‘IM8’, he has started marketing two supplements (one of his slogans is ‘Built by Science, Trusted by Beckham’):
Daily Ultimate Essentials: All-in-One Supplement
This is a ‘multi-everything’ supplement. The only truly remarkable thing about it is its price tag. There are hundreds of similar products on the market. Almost all of them are much cheaper, and none is helpful for anyone who is healthy and consumes a balanced diet, as far as I can see.
Daily Ultimate Longevity: Healthy Aging
The implication here seems to be not a trivial one; the name clearly implies that we live longer, if we regularly bought this supplement. Not onlly that, we would also be healthier! I can see no evidence for either of these claims, yet a simple calculation tells me that we would be considerably poorer, if we fell for this advertising gimmick.
On the website, we learn a bit more:
At IM8, our commitment to science goes beyond innovation—it’s the foundation of everything we do. A world-class team of experts from space science, medicine, and academia has united with one goal: to revolutionize wellness. We’ve pioneered CRT8™ (Cell Rejuvenation Technology 8), designed to enhance cellular rejuvenation and push the limits of what’s possible in health.
Each of our products undergoes rigorous third-party testing and clinical trials, ensuring purity, efficacy, and results you can trust. With IM8, you’re getting scientifically driven core nutrition for optimal health and longevity.
___________________
I feel embarrassed for the ‘world-class team of experts from space science, medicine, and academia’ who give their good name to this hyped up nonsense. Moreover, I ask myself whether David Beckham’s new attempt to increase his wealth might be a case for the Advertising Standards Authority (ASA).
“As a medical doctor having taken care of thousands of patients in my life, I strive to ensure the health safety and superior wellbeing of my patients. I continue to encourage, educate and inform not only my patients, but the public to stay strong and healthy any time, not just during a pandemic. Our body is our temple and what we put in it and what we don’t affects the way we feel, think and function. Essential vitamins and minerals are key component to daily functioning but thats not always possible in this day and age with our busy hectic lifestyles, so after years of educating my patients, now I made it a little easier to get all the nutrition you need to live strong and stay healthy.”
These are the words from an advertisement for “Immune System Support for your Active Life” sold by Dr. Janette Nesheiwat who was just nominated as Donald Trump’s next SURGEON GENERAL. Amongst other items, she sells 60 capsules of ‘B+C BOOST Plus D3 & Zinc‘ for US$26.99.
Her website describes the new US Surgeon General as follows:
Dr. Janette Nesheiwat is a top Family and Emergency Medicine doctor. She brings a refreshingly no-nonsense attitude to the latest medical news, breaking down everything you need to know to keep you- and your family- healthy at all times.
Whether caring for her patients in the ER, serving on the front lines of disaster relief with the Red Cross, or sharing need-to-know info with TV audiences, Dr. Nesheiwat’s mission is not only to save lives—but to change them, by giving real people the treatment and the expertise they need.
Her sincere and straightforward approach is a product of her background. She was one of five kids raised by a widowed mother, and also completed US Army ROTC Advanced Officer Training in Ft. Lewis, Washington prior to becoming a Family and Emergency Physician. She has led medical relief missions around the globe and today she is a medical news correspondent and the Medical Director at CityMD.
I was always telling my patients who were unwell drink some tea, take some vitamin b12 and vitamin C. I found myself repeating my all natural regimen to my patients over and over “take some B12 and C to Boost” your immune system. Thats how I came up with BC Boost. Although I am a doctor, I am not quick to prescribe drugs unless I feel necessary as we want to put into our body the most natural wholesome ingredients.
Vitamin B12 is a cofactor in DNA synthesis. It helps maintain healthy blood cells and nerve function as well as prevent anemia which causes fatigue, a common complaint in those who are sick, tired, run down. Vitamin C is needed for development of collagen and a strong immune system as well as body repair and growth.
Yes, you are quite right, Dr. Nesheiwat might have forgotten one or two not-so-unimportant details:
- If you eat a healthy diet, you don’t need vitamins.
- If you do need vitamins, you can buy them cheaper elsewhere.
- These vitamins do not boost your immune system.
- Boosting the immune system could actually do a lot of harm to the many people suffering from auto-immune diseases.
But never mind, we can nevertheless be confident that Dr. Nesheiwat will bring great joy to the US supplement industry. I am less confident, however, that she did public health a great service when, in her role as a regular ‘Fox News’ commentator, she warned that wearing face masks during the pandemic exposed consumers to toxic substances linked to seizures and cancer.
Drip IV is “Australia’s first and leading mobile healthcare company specialising in assisting with nutritional deficiencies”. They claim to provide a mobile IV service that is prescribed and tailored individually to your nutritional needs. Treatment plans and customised infusions are determined by a medical team to suit individual requirements. They deliver vitamins, minerals and amino acids directly to the body via the bloodstream, a method they state allows for optimal bioavailability.
These claims are a little puzzling to me, not least because vitamins, minerals and amino acids tailored individually to the nutritional needs of the vast majority of people would mean administering nothing at all. But I guess that virtually every person who consults the service will get an infusion [and pay dearly for it].
The Australian Therapeutic Goods Administration (TGA) seems to have a similarly dim view on Drip IV. The TGA has just issued 20 infringement notices totalling $159,840 to the company and to one of its executive officers. The reason: unlawful advertising of intravenous infusion products to Australian consumers on a company website and social media. Ten notices totalling $133,200 were issued to the company and ten notices totalling $26,640 were issued to an executive officer. The TGA considers the intravenous infusion products to be therapeutic goods because of the claims made about them, and the advertising to be unlawful because the advertisements allegedly:
- contained prohibited representations, such as claims regarding cancer.
- contained restricted representations such as that the products would alleviate fatigue caused by COVID-19, assist in the treatment of Graves’ Disease and Alzheimer’s Disease, and support the treatment of autoimmune diseases such as Multiple Sclerosis. No TGA approval had been given to make such claims.
- referred to ingredients that are prescription only, such as glutathione. Prescription medicines cannot be advertised directly to the public in Australia.
- contained a statement or picture suggesting or implying the products were ‘TGA Approved’. Advertising of therapeutic goods cannot include a government endorsement.
- contained a statement or picture expressing that the goods were ‘miraculous’.
Vitamin infusions have become very popular around the globe. There are now thousands of clinics offering this service, and many of them advertise aggressively with claims that are questionable. Here is just one example from the UK:
Modern life is hectic. If you are looking to boost your wellbeing, increase your energy levels, lift your mood and hydrate your body, Vitamin IV Infusions are ideal. Favoured by celebrities such as Madonna, Simon Cowell and Rihanna, Vitamin IV Infusions are an easy, effective way of delivering vitamins, minerals and amino acids directly into your bloodstream via an IV (intravenous) drip. Vitamins are essential for normal growth and staying healthy – but our bodies can’t produce all of the nutrients we need to function and thrive. That’s why more than one in three people take daily vitamin supplements – often without realising that only 15% of the active nutrients consumed orally actually find their way into their bloodstream. With Vitamin IV Infusions, the nutrients enter your bloodstream directly and immediately, and are delivered straight to your cells. We offer four different Vitamin IV Infusions, so you can choose the best combination for your personal needs, while boosting your general health, energy and wellbeing.
My advice to consumers is a little different and considerably less costly:
- to ensure you get enough vitamins, minerals, and amino acids, eat a balanced diet;
- to boost your well-being, sit down and calculate the savings you made by NOT using such a service;
- to increase your energy levels, take a nap;
- to lift your mood, recount the money you saved and think of what nice things you might buy with it;
- to hydrate your body drink a glass of water.
Perhaps it is time the authorities in all countries had a look at what these clinics are offering and what health claims they are making. Perhaps it is time they act as the TGA just did.
The U.S. Food and Drug Administration issued warning letters to seven companies for illegally selling dietary supplements that claim to cure, treat, mitigate or prevent cardiovascular disease or related conditions, such as atherosclerosis, stroke or heart failure, in violation of the Federal Food, Drug, and Cosmetic Act (FD&C Act). The FDA is urging consumers not to use these or similar products because they have not been evaluated by the FDA to be safe or effective for their intended use and may be harmful.
The warning letters were issued to:
- Essential Elements (Scale Media Inc.);
- Calroy Health Sciences LLC;
- Iwi;
- BergaMet North America LLC;
- Healthy Trends Worldwide LLC (Golden After 50);
- Chambers’ Apothecary;
- Anabolic Laboratories, LLC.
“Given that cardiovascular disease is the leading cause of death in the U.S., it’s important that the FDA protect the public from products and companies that make unlawful claims to treat it. Dietary supplements that claim to cure, treat, mitigate or prevent cardiovascular disease and related conditions could potentially harm consumers who use these products instead of seeking safe and effective FDA-approved treatments from qualified health care providers,” said Cara Welch, Ph.D., director of the Office of Dietary Supplement Programs in the FDA’s Center for Food Safety and Applied Nutrition. “We encourage consumers to remain vigilant when shopping online or in stores to avoid purchasing products that could put their health at risk.”
Under the FD&C Act, products intended to diagnose, cure, treat, mitigate or prevent disease are drugs and are subject to the requirements that apply to drugs, even if they are labeled as dietary supplements. Unlike drugs approved by the FDA, the agency has not evaluated whether the unapproved products subject to the warning letters announced today are effective for their intended use, what the proper dosage might be, how they could interact with FDA-approved drugs or other substances, or whether they have dangerous side effects or other safety concerns.
The FDA advises consumers to talk to their doctor, pharmacist or other health care provider before deciding to purchase or use any dietary supplement or drug. Some supplements might interact with medicines or other supplements. Health care providers will work with patients to determine which treatment is the best option for their condition.
If a consumer thinks that a product might have caused a reaction or an illness, they should immediately stop using the product and contact their health care provider. The FDA encourages health care providers and consumers to report any adverse reactions associated with FDA-regulated products to the agency using MedWatch or the Safety Reporting Portal.
The FDA has requested responses from the companies within 15 working days stating how they will address the issues described in the warning letters or provide their reasoning and supporting information as to why they think the products are not in violation of the law. Failure to correct violations promptly may result in legal action, including product seizure and/or injunction.
Dietary supplements are touted for cognitive protection, but supporting evidence is mixed. COSMOS-Mind tested whether daily administration of cocoa extract (containing 500 mg/day flavanols) versus placebo and a commercial multivitamin-mineral (MVM) versus placebo improved cognition in older women and men.
COSMOS-Mind, a large randomized two-by-two factorial 3-year trial, assessed cognition by telephone at baseline and annually. The primary outcome was a global cognition composite formed from mean standardized (z) scores (relative to baseline) from individual tests, including the Telephone Interview of Cognitive Status, Word List and Story Recall, Oral Trail-Making, Verbal Fluency, Number Span, and Digit Ordering. Using intention-to-treat, the primary endpoint was change in this composite with 3 years of cocoa extract use. The pre-specified secondary endpoint was change in the composite with 3 years of MVM supplementation. Treatment effects were also examined for executive function and memory composite scores, and in pre-specified subgroups at higher risk for cognitive decline.
A total of 2262 participants were enrolled (mean age = 73y; 60% women; 89% non-Hispanic White), and 92% completed the baseline and at least one annual assessment. Cocoa extract had no effect on global cognition (mean z-score = 0.03, 95% CI: -0.02 to 0.08; P = .28). Daily MVM supplementation, relative to placebo, resulted in a statistically significant benefit on global cognition (mean z = 0.07, 95% CI 0.02 to 0.12; P = .007), and this effect was most pronounced in participants with a history of cardiovascular disease (no history: 0.06, 95% CI 0.01 to 0.11; history: 0.14, 95% CI -0.02 to 0.31; interaction, nominal P = .01). Multivitamin-mineral benefits were also observed for memory and executive function. The cocoa extract by MVM group interaction was not significant for any of the cognitive composites.
The authors concluded that the Cocoa extract did not benefit cognition. However, COSMOS-Mind provides the first evidence from a large, long-term, pragmatic trial to support the potential efficacy of a MVM to improve cognition in older adults. Additional work is needed to confirm these findings in a more diverse cohort and to identify mechanisms to account for MVM effects.
This trial certainly has a few stunning features. For instance, its sample size was impressive and its follow-up period long. But it also has a few weak points. The study was conducted remotely via mail or telephone which means that compliance was impossible to control. Moreover, the outcome measures were subjective, and blinding was not checked. In addition, I fail to see a plausible mechanism of action. Most importantly, the generalizability of the results to the population at large seems questionable. It might make sense that older individuals many of whom might have low vitamin levels can profit from MVM. Whether this is also true for younger people who are well-nourished might be a different matter.
Even though most people do not think about it in this way, tea is a herbal remedy. We know that it is pleasant, but is it also effective?
This study explored the associations between tea drinking and the incident risk of type 2 diabetes mellitus(T2 DM). A dynamic prospective cohort study among a total of 27 841 diabetes-free permanent adult residents randomly selected from 2, 6, and 7 rural communities between 2006-2008, 2011-2012, and 2013-2014, respectively. Questionnaire survey, physical examination, and laboratory test were carried out among the participants. In 2018, the researchers conducted a follow-up through the electronic health records of residents. Cox regression models were applied to explore the association between tea drinking and the incident risk of T2 DM and estimate the hazard ratio(HR), and its 95%CI.
Among the 27 841 rural community residents in Deqing County, 10 726(39%) were tea drinkers, 8215 (77%) of which were green tea drinkers. A total of 883 new T2 DM incidents were identified until December 31, 2018, and the incidence density was 4.43 per 1000 person-years (PYs). The incidence density was 4.07/1000 PYs in those with tea drinking habits and 4.71/1000 PYs in those without tea drinking habits. The incidence density was 3.79/1000 PYs in those with green tea drinking habits. After controlling for sex, age, education, farming, smoking, alcohol consumption, dietary preference, body mass index, hypertension, impaired fasting glucose, and family history of diabetes, the risk of T2 DM among rural residents with tea drinking habits was 0.79 times higher than that among residents without tea drinking habits(HR=0.79, 95%CI 0.65-0.96), and the risk of T2 DM among residents with green tea drinking habits was 0.72 times higher than that among residents without tea drinking habits(HR=0.72, 95%CI 0.58-0.89). No significant associations were found between other kinds of tea and the risk of T2 DM, nor the amount of green tea-drinking.
The authors concluded that drinking green tea may reduce the risk of T2 DM among adult population in rural China.
Epidemiological studies of this nature resemble big fishing expeditions that can bring up all sorts of rubbish and – if lucky – also some fish. The question thus is whether this study identified an interesting association or just some odd rubbish.
A quick look into Medline seems to suggest great caution. Here are the conclusions from a few further case-control studies:
- In Chinese adults, daily green tea consumption was associated with a lower risk of incident T2D and a lower risk of all-cause mortality in patients with diabetes, but the associations for other types of tea were less clear. In addition, daily tea consumption was associated with a lower risk of diabetic microvascular complications, but not macrovascular complications.
- Green tea drinking was associated with an increased risk of T2D in Chinese adults. The mechanisms underlying the association need to be elucidated.
- Tea consumers had reduced risks of all-cause mortality and partial cause-specific mortality, but not for the risk of death from cancer. On the contrary, daily tea drinkers with smoking habits and excessive alcohol drinking had an increased risk of death from cancer.
Thus the question of whether tea drinking might prevent diabetes remains open, in my view.
Yet, the paper might teach us two important lessons:
- Case-control studies must be taken with a pinch of salt.
- Correlation is not the same as causation.
This study aimed to evaluate the efficacy of Persian barley water in controlling the clinical outcomes of hospitalized COVID-19 patients. It was designed as a single-blind, add-on therapy, randomized controlled clinical trial and conducted in Shiraz, Iran, from January to March 2021. One hundred hospitalized COVID-19 patients with moderate disease severity were randomly allocated to receive routine treatment (per local protocols) with or without 250 ml of Persian barley water (PBW) daily for two weeks. Clinical outcomes and blood tests were recorded before and after the study period. Multivariable modeling was applied using Stata software for data analysis.
The length of hospital stay (LHS) was 4.5 days shorter in the intervention group than the control group regardless of history of cigarette smoking (95% confidence interval: -7.22, -1.79 days). Also, body temperature, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and creatinine significantly dropped in the intervention group compared to the control group. No adverse events related to PBW occurred.
The authors from the Department of Traditional Medicine, Shiraz University of Medical Sciences, Shiraz, Iran, concluded that this clinical trial demonstrated the efficacy of PBW in minimizing the LHS, fever, and levels of ESR, CRP, and creatinine among hospitalized COVID-19 patients with moderate disease severity. More robust trials can help find safe and effective herbal formulations as treatments for COVID-19.
I must admit, I did not know about PBW. The authors explain that PBW is manufactured from Hordeum vulgare via a specific procedure. According to recent studies, barley is rich in constituents such as selenium, tocotrienols, phytic acid, catechin, lutein, vitamin E, and vitamin C; these compounds are responsible for their antioxidant and anti-inflammatory properties. Barley grains also have immune-stimulating effects, antioxidant properties, protective effects on the liver and digestive systems, anti-cancer effects, and act to reduce uric acid levels.
But even if these effects would constitute a plausible mechanism for explaining the observed effects (which I do not think they do), the study itself is more than flimsy.
I do not understand why researchers investigating an important issue do not make sure that their study is as rigorous as possible.
- Why not use an adequately large sample size?
- Why not employ a placebo?
- Why not double-blind?
- Why not report the most important outcome, i.e. mortality?
As it stands, nobody will take this study seriously. Perhaps this is a good thing – but perhaps PBW does have positive effects (I know it’s a long shot) and, in this case, a poor-quality study would only prevent an effective therapy come to light.
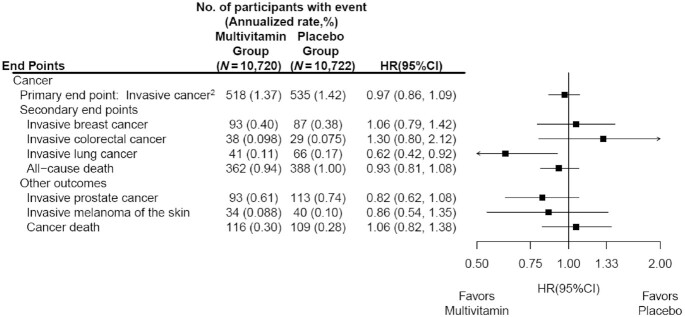
Many older adults commonly take multivitamin-multimineral (MVM) supplements to promote health. Yet, evidence on the use of daily MVMs on invasive cancer is limited.
The objective of this study was therefore to determine if a daily MVM decreases total invasive cancer among older adults. For this purpose, a team of researchers performed a randomized, double-blind, placebo-controlled, 2-by-2 factorial trial of a daily MVM and cocoa extract for prevention of cancer and cardiovascular disease (CVD) among 21,442 US adults (12,666 women aged ≥65 y and 8776 men aged ≥60 y) free of major CVD and recently diagnosed cancer. The intervention phase was from June 2015 through December 2020. This article reports on the MVM intervention.
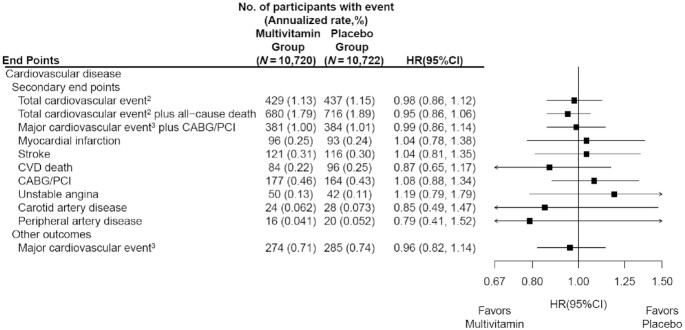
Participants were randomly assigned to daily MVM or placebo. The primary outcome was total invasive cancer, excluding nonmelanoma skin cancer. Secondary outcomes included major site-specific cancers, total CVD, all-cause mortality, and total cancer risk among those with a baseline history of cancer.
During a median follow-up of 3.6 y, invasive cancer occurred in 518 participants in the MVM group and 535 participants in the placebo group (HR: 0.97; 95% CI: 0.86, 1.09; P = 0.57). No significant effect was observed of a daily MVM on breast cancer (HR: 1.06; 95% CI: 0.79, 1.42) or colorectal cancer (HR: 1.30; 95% CI: 0.80, 2.12). The researchers observed a protective effect of a daily MVM on lung cancer (HR: 0.62; 95% CI: 0.42, 0.92). The composite CVD outcome occurred in 429 participants in the MVM group and 437 participants in the placebo group (HR: 0.98; 95% CI: 0.86, 1.12). MVM use did not significantly affect all-cause mortality (HR: 0.93; 95% CI: 0.81, 1.08). There were no safety concerns.
The authors concluded that a daily MVM supplement, compared with placebo, did not significantly reduce the incidence of total cancer among older men and women. Future studies are needed to determine the effects of MVMs on other aging-related outcomes among older adults.
This is an excellent and important study with clear findings. Nevertheless, the authors insist that several limitations should be considered. First, the COSMOS intervention was relatively short to detect a potential small-to-moderate effect on cancer outcomes given the long duration of time typically required for nutritional interventions to potentially reduce cancer risk. Second, the secondary and exploratory analyses should be interpreted with caution, especially given an overall lack of effect of an MVM on the primary outcome of total invasive cancer. Third, the authors successfully leveraged existing cohorts with mass mailings to expedite recruitment and randomization of 21,442 participants into COSMOS. However, generalizability may be limited, with modest diversity of 10% non-Whites and 2.6% Hispanics plus healthy volunteer bias for participants willing and eligible to enroll in a mail-based clinical trial.
The US Food and Drug Administration (FDA) state that dietary supplements can help people improve or maintain their overall health. But they may also come with health risks. Whether you’re a consumer of dietary supplements or it’s your job to inform and educate, it’s important to know the facts before deciding to take any dietary supplement.
Therefore, they launched the initiative, “Supplement Your Knowledge”. It aims to help inform health care professionals, consumers, and educators about dietary supplements.
“Dietary supplements can be valuable to your health but taking some supplements can also involve health risks,” Douglas Stearn, JD, deputy director for regulatory affairs in the FDA’s Center for Food Safety and Applied Nutrition, said in a statement. “These Supplement Your Knowledge resources will help provide consumers and health care professionals with facts to make informed decisions when determining if they want to use or recommend dietary supplements.”
In collaboration with the American Medical Association, publisher of JAMA, the FDA has developed a free continuing medical education program for physicians and other health care professionals about the regulation of dietary supplements, informing patients about their use, and reporting adverse events to the agency. The program includes 3 videos and accompanying educational materials. It is available on the FDA website and the AMA Ed Hub.
________________________
The objectives of the program are:
1. Define dietary supplements
2. Describe how dietary supplements are regulated
3. Describe how dietary supplements are labelled and the types of claims permitted
4. Review potential interactions of dietary supplements with other supplements, medications, and laboratory tests
5. Identify adverse events and how to report them to FDA
Even though some parts of the program are quite specific to the US, I think that the initiative is most laudable and an excellent resource for physicians, SCAM practitioners, consumers, and decision-makers to learn more about this important subject.
The US Food and Drug Administration created the Tainted Dietary Supplement Database in 2007 to identify dietary supplements adulterated with active pharmaceutical ingredients (APIs). This article compared API adulterations in dietary supplements from the 10-year time period of 2007 through 2016 to the most recent 5-year period of 2017 through 2021. Its findings are alarming:
- From 2007 through 2021, 1068 unique products were found to be adulterated with APIs.
- Sexual enhancement and weight-loss dietary supplements are the most common products adulterated with APIs.
- Phosphodiesterase-5 inhibitors are commonly included in sexual enhancement dietary supplements.
- A single product can include up to 5 APIs.
- Sibutramine, a drug removed from the market due to cardiovascular adverse events, is the most included adulterant API in weight loss products.
- Sibutramine analogues, phenolphthalein (which was removed from the US market because of cancer risk), and fluoxetine were also included.
- Muscle-building dietary supplements were commonly adulterated before 2016, but since 2017 no additional adulterated products have been identified.
The authors concluded that the lack of disclosure of APIs in dietary supplements, circumventing the normal procedure with clinician oversight of prescription drug use, and the use of APIs that are banned by the Food and Drug Administration or used in combinations that were never studied are important health risks for consumers.
The problem of adulterated supplements is by no means new. A similar review published 4 years ago already warned that “active pharmaceuticals continue to be identified in dietary supplements, especially those marketed for sexual enhancement or weight loss, even after FDA warnings. The drug ingredients in these dietary supplements have the potential to cause serious adverse health effects owing to accidental misuse, overuse, or interaction with other medications, underlying health conditions, or other pharmaceuticals within the supplement.”
These papers relate to the US where supplement use is highly prevalent. The harm done by adulterated products is thus huge. If we focus on Chinese or Ayurvedic supplements, the problem might even be more serious. In 2002, my own review concluded that adulteration of Chinese herbal medicines with synthetic drugs is a potentially serious problem which needs to be addressed by adequate regulatory measures. Twenty years later, we seem to be still waiting for effective regulations that protect the consumer.
Progress in medicine, they say, is made funeral by funeral!