Developing interventions against age-related memory decline and for older adults experiencing neurodegenerative disease is perhaps one of the greatest challenges of our generation. Spermidine supplementation has shown beneficial effects on brain and cognitive health in animal models, and there has been preliminary evidence of memory improvement in individuals with subjective cognitive decline.
This randomized, double-masked, placebo-controlled phase 2b trial was aimed at determining the effect of longer-term spermidine supplementation on memory performance and biomarkers in this at-risk group. The study was a monocenter trial carried out at an academic clinical research center in Germany. Eligible individuals were aged 60 to 90 years with subjective cognitive decline who were recruited from health care facilities as well as through advertisements in the general population.
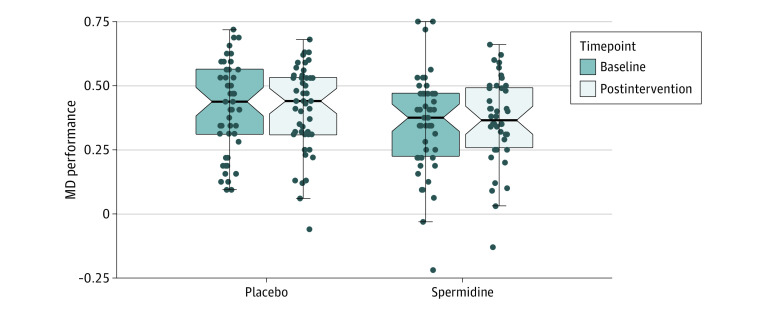
One hundred participants were randomly assigned (1:1 ratio) to 12 months of dietary supplementation with either a spermidine-rich dietary supplement extracted from wheat germ (0.9 mg spermidine/d) or placebo (microcrystalline cellulose). Eighty-nine participants (89%) successfully completed the trial. The primary outcome was change in memory performance from baseline to 12-month postintervention assessment (intention-to-treat analysis), operationalized by mnemonic discrimination performance assessed by the Mnemonic Similarity Task. Secondary outcomes included additional neuropsychological, behavioral, and physiological parameters. Safety was assessed in all participants and exploratory per-protocol, as well as subgroup, analyses were performed.
A total of 100 participants (51 in the spermidine group and 49 in the placebo group) were included in the analysis (mean [SD] age, 69 [5] years; 49 female participants [49%]). Over 12 months, no significant changes were observed in mnemonic discrimination performance (between-group difference, -0.03; 95% CI, -0.11 to 0.05; P = .47) and secondary outcomes. Exploratory analyses indicated possible beneficial effects of the intervention on inflammation and verbal memory. Adverse events were balanced between groups.
The authors concluded that in this randomized clinical trial, longer-term spermidine supplementation in participants with subjective cognitive decline did not modify memory and biomarkers compared with placebo. Exploratory analyses indicated possible beneficial effects on verbal memory and inflammation that need to be validated in future studies at higher dosage.
The absence of an effect might have, according to the authors, two reasons.
- The daily dose of 0.9 mg spermidine might not have been sufficient to achieve strong effects on memory function and biomarkers in cognitively healthy older individuals.
- The supplementation with dietary spermidine might not act as a memory booster, but rather prevent age-related memory impairment and development of AD, a possibility supported by evidence from animal studies.
I am tempted to add a third one: spermidine might not be effective at all for this indication (or any other condition)!

Leave a Reply