clinical trial
Horticultural therapy (HT)?
What on earth is that?
Don’t worry, it was new to me too and I first thought of the treatment of plants.
HT is said to be a “time-proven practice. The therapeutic benefits of garden environments have been documented since ancient times. In the 19th century, Dr. Benjamin Rush, a signer of the Declaration of Independence and recognized as the “Father of American Psychiatry,” was first to document the positive effect working in the garden had on individuals with mental illness. In the 1940s and 1950s, rehabilitative care of hospitalized war veterans significantly expanded acceptance of the practice. No longer limited to treating mental illness, horticultural therapy practice gained in credibility and was embraced for a much wider range of diagnoses and therapeutic options. Today, horticultural therapy is accepted as a beneficial and effective therapeutic modality. It is widely used within a broad range of rehabilitative, vocational, and community settings. Horticultural therapy techniques are employed to assist participants to learn new skills or regain those that are lost. Horticultural therapy helps improve memory, cognitive abilities, task initiation, language skills, and socialization. In physical rehabilitation, horticultural therapy can help strengthen muscles and improve coordination, balance, and endurance. In vocational horticultural therapy settings, people learn to work independently, problem solve, and follow directions. Horticultural therapists are professionals with specific education, training, and credentials in the use of horticulture for therapy and rehabilitation. Read the formal definition of the role of horticultural therapists.”
As always, the question is DOES IT WORK?
This systematic review and meta-analysis aimed to evaluate HT for general health in older adults. Electronic databases as well as grey literature databases, and clinical trials registers were searched from inception to March 2021. Randomized controlled trials (RCTs), quasi-RCTs (QRCTs), and cohort studies about HT for adults aged over 60 were included in this review. Outcome measures were physical function, quality of life, BMI, mood tested by self-reported questionnaire and the expression of the immune cells.
Fifteen studies (thirteen RCTs and two cohort studies) involving 1046 older participants were included. Meta-analysis showed that HT resulted in better quality of life (MD 2.09, 95% CI [1.33, 2.85], P<0. 01) and physical function (SMD 0.82, 95% [0.36, 1.29], P<0.01) compared with no-gardener; the similar findings showed in BMI (SMD -0.30, 95% [-0.57, -0.04], P = 0.02) and mood tested by self-reported questionnaire (SMD 2.80, 95% CI [1.82, 3.79], P<0. 01). And HT might be beneficial for blood pressure and immunity, while all the evidence was moderate-quality judged by GRADE.
The authors concluded that HT may improve physical function and quality of life in older adults, reduce BMI and enhance positive mood. A suitable duration of HT may be between 60 to 120 minutes per week lasting 1.5 to 12 months. However, it remains unclear as to what constitutes an optimal recommendation.
I have considerable problems with this review and its conclusion:
- It is simply untrue that there were 13 RCTs; several of these studies were clearly not randomized.
- Most of the studies are of very poor quality. For instance, they often did not make the slightest attempt to control for non-specific effects, yet they concluded that the observed outcome was a specific effect of HT.
My biggest problem does, however, not relate to methodological issues. My main issue with this paper is one of definition. What is a ‘therapy’ and what not? If we call a bit of gardening a ‘therapy’ are we not descending to the level of those who call a bit of shopping ‘retail therapy’? To put it differently, is HT superior to retail therapy? And do we need RCTs to answer this question?
What is wrong with encouraging people who like gardening to just do it? I, for instance, like drumming; but I do not believe we need a few RCTs to prove that it is healthy. Not every past-time or hobby that makes you feel good is a therapy and needs to be scrutinized as such.
Yes, Today is ‘WORLD SLEEP DAY‘ and you are probably in bed hoping this post will put you back to sleep.
This study aimed to synthesise the best available evidence on the safety and efficacy of using moxibustion and/or acupuncture to manage cancer-related insomnia (CRI).
The PRISMA framework guided the review. Nine databases were searched from its inception to July 2020, published in English or Chinese. Randomised clinical trials (RCTs) of moxibustion and or acupuncture for the treatment of CRI were selected for inclusion. The methodological quality was assessed using the method suggested by the Cochrane collaboration. The Cochrane Review Manager was used to conduct a meta-analysis.
Fourteen RCTs met the eligibility criteria; 7 came from China. Twelve RCTs used the Pittsburgh Sleep Quality Index (PSQI) score as continuous data and a meta-analysis showed positive effects of moxibustion and or acupuncture (n = 997, mean difference (MD) = -1.84, 95% confidence interval (CI) = -2.75 to -0.94, p < 0.01). Five RCTs using continuous data and a meta-analysis in these studies also showed significant difference between two groups (n = 358, risk ratio (RR) = 0.45, 95% CI = 0.26-0.80, I 2 = 39%).
The authors concluded that the meta-analyses demonstrated that moxibustion and or acupuncture showed a positive effect in managing CRI. Such modalities could be considered an add-on option in the current CRI management regimen.
Even at the risk of endangering your sleep, I disagree with this conclusion. Here are some of my reasons:
- Chinese acupuncture trials invariably are positive which means they are as reliable as a 4£ note.
- Most trials were of poor methodological quality.
- Only one made an attempt to control for placebo effects.
- Many followed the A+B versus B design which invariably produces (false-) positive results.
- Only 4 out of 14 studies mentioned adverse events which means that 10 violated research ethics.
Sorry to have disturbed your sleep!
This review assessed the magnitude of reporting bias in trials assessing homeopathic treatments and its impact on evidence syntheses.
A cross-sectional study and meta-analysis. Two persons independently searched Clinicaltrials.gov, the EU Clinical Trials Register and the International Clinical Trials Registry Platform up to April 2019 to identify registered homeopathy trials. To determine whether registered trials were published and to detect published but unregistered trials, two persons independently searched PubMed, Allied and Complementary Medicine Database, Embase and Google Scholar up to April 2021. For meta-analyses, the authors used random effects models to determine the impact of unregistered studies on meta-analytic results.
The investigators reported the proportion of registered but unpublished trials and the proportion of published but unregistered trials. They also assessed whether primary outcomes were consistent between registration and publication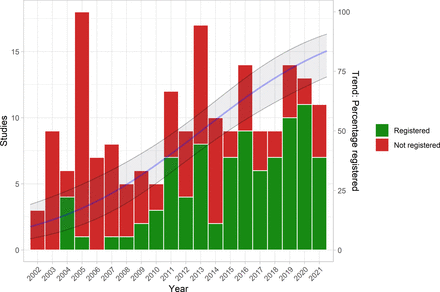
Since 2002, almost 38% of registered homeopathy trials have remained unpublished, and 53% of published randomised controlled trials (RCTs) have not been registered. Retrospective registration was more common than prospective registration. Furthermore, 25% of primary outcomes were altered or changed compared with the registry. Although we could detect a statistically significant trend toward an increase of registrations of homeopathy trials (p=0.001), almost 30% of RCTs published during the past 5 years had not been registered.
A meta-analysis stratified by registration status of RCTs revealed substantially larger treatment effects of unregistered RCTs (SMD: −0.53, 95% CI −0.87 to −0.20) than registered RCTs (SMD: −0.14, 95% CI −0.35 to 0.07).
The authors concluded that registration of published trials was infrequent, many registered trials were not published and primary outcomes were often altered or changed. This likely affects the validity of the body of evidence of homeopathic literature and may overestimate the true treatment effect of homeopathic remedies.
An obvious investigation to do (why did I not have this idea?)!
And a finding that will surprise few (except fans of homeopathy who will, of course, dispute it).
The authors also mention that reporting biases are likely to have a substantial impact on the estimated treatment effect of homeopathy. Using data from a highly cited meta-analysis of homeopathy RCTs, our example showed that unregistered trials yielded substantially larger treatment effects than registered trials. They also caution that, because of the reporting biases identified in their analysis, effect estimates of meta-analyses of homeopathy trials might substantially overestimate the true treatment effect of homeopathic remedies and need to be interpreted cautiously.
In other words, the few reviews suggesting that homeopathy works beyond placebo (and are thus celebrated by homeopaths) are most likely false-positive. And the many reviews showing that homeopathy does not work would demonstrate this fact even clearer if the reporting bias had been accounted for.
Or, to put it bluntly:
The body of evidence on homeopathy is rotten to the core and therefore not reliable.
Plantar fasciitis (PF) is a chronic degenerative condition causing marked thickening and fibrosis of the plantar fascia, and collagen necrosis, chondroid metaplasia and calcification. There is little convincing evidence in support of various approaches, including homeopathy, for treating PF. This study was undertaken to examine the efficacy of individualized homeopathic medicines (IHMs) compared with placebo in the treatment of PF.
This double-blind, randomized, placebo-controlled trial was conducted at the outpatient departments of Mahesh Bhattacharyya Homoeopathic Medical College and Hospital, West Bengal, India. Patients were randomized to receive either IHMs or identical-looking placebo in the mutual context of conservative non-medicinal management. The Foot Function Index (FFI) questionnaire, as an outcome measure, was administered at baseline, and every month, up to 3 months. Group differences (unpaired t-tests) and effect sizes (Cohen’s d) were calculated on an intention-to-treat sample. The sample was analyzed statistically after adjusting for baseline differences.
The target sample size was 128; however, only 75 could be enrolled (IHMs: 37; Placebo: 38). Attrition rate was 9.3% (IHMs: 4, Placebo: 3). Differences between groups in total FFI% score favored IHMs against placebo at all the time points, with large effect sizes: month 1 (mean difference, -10.0; 95% confidence interval [CI], -15.7 to -4.2; p = 0.001; d = 0.8); month 2 (mean difference, -14.3; 95% CI, -20.4 to -8.2; p <0.001; d = 1.1); and month 3 (mean difference, -23.3; 95% CI, -30.5 to -16.2; p <0.001; d = 1.5). Similar significant results were also observed on three FFI sub-scales (pain%, disability%, and activity limitation%). Natrum muriaticum (n = 14; 18.7%) and Rhus toxicodendron and Ruta graveolens (n = 11 each; 14.7%) were the most frequently prescribed medicines. No harms, serious adverse events, or intercurrent illnesses were recorded in either of the groups.
The authors concluded that IHMs acted significantly better than placebo in the treatment of PF; however, the trial being underpowered, the results should be interpreted as preliminary only. Independent replications are warranted.
It is nice to see homeopaths stress the importance of independent replication. It is less nice, however, to note their main conclusion:
IHMs acted significantly better than placebo.
This essentially is what will stick in the minds of the pro-homeopathy reader, and this is the information that will enter into future meta-analyses and systematic reviews of homeopathy. But this is also untrue! The qualifier that follows is but a lame excuse for drawing a wrong conclusion. In my view, a correct conclusion would read something like this:
Our study failed to recruit a sufficient number of patients. Therefore, no conclusions about the efficacy of IHM can be drawn from it.
The present study investigated the impact of a purposefully designed Islamic religion-based intervention on reducing depression and anxiety disorders among Muslim patients using a randomised controlled trial design. A total of 62 Muslim patients (30 women and 32 men) were divided by gender into two groups, with each group assigned randomly to either treatment or control groups. The participants who received the Islamic-based intervention were compared to participants who received the control intervention.
The Islamic-Based Intervention that was applied to the two experimental groups (i.e. one male, one female) has several components. These components were based on moral and religious concepts and methods, including moral confession, repentance, insight, learning, supplication, seeking Allah’s mercy, seeking forgiveness, remembrance of Allah, patience, trust in Allah, self-consciousness, piety, spiritual values, and moral principles. The techniques implemented in the intervention included the art of asking questions, clarifying, listening, interacting, summarising, persuading, feedback, empathy, training practice, reflecting feelings, discussion, and dialogue, lecturing, brainstorming, reinforcement, modeling, positive self-talk, evaluation, homework, practical applications, activation games (play through activities), emotional venting, stories, presentation, correction of thoughts, and relaxation. The two control groups (i.e. one male, one female) received the energy path program provided by the Al-Nour Centre. This program aimed to enhance self-confidence and modify people’s behavior with anxiety disorders, depression, and obsessive-compulsive disorder. Both interventions comprised 30 sessions over 30 h; two sessions were conducted per week, and each session lasted for 60 min (one hour). The duration of the intervention was 15 weeks.
Taylor’s manifest anxiety scale and Steer and Beck’s depression scale were used for examining the effects on depression and anxiety levels. The results revealed that the Islamic intervention significantly reduced anxiety levels in women and depression levels in men compared to the typical care control groups.
The authors concluded that religious intervention played a vital role in lowering the patients’ level of anxiety among women and depression among men. In general, religious practices prevent individuals from becoming subject to mental disorders, i.e. anxiety and depression.
The authors comment that the Islamic religion-based intervention (RSAFI) significantly reduced the levels of depression and anxiety among the participants. Also, there was a substantial improvement in the patients’ general health after the intervention. They were satisfied and believed that everything happening to them was destined by Allah. These results could be attributed to the different intervention practices that relied on the guidance of the Holy Quran and Sunnah. For instance, Saged et al. (2020) confirmed that the Holy Quran significantly impacts healing patients who suffer from physical, psychological, and mental disorders. In this respect, Moodley et al. (2018) concluded that having faith in Allah offers a relatively quick approach to healing patients suffering from heartache and depression. This goes hand in hand because the recitation of the Quran and remembrance of Allah help patients feel relaxed and peaceful. Muslims believe that the Quran is the word of Allah and that Allah’s words exert a significant impact on the healing of mental health patients, as, ultimately, Almighty Allah is the one who cures illnesses.
When discussing the limitations of their study, the authors state that the sample of this study was limited to the patients with anxiety and depression disorders at the Al-Nour Centre in Kuala Lumpur, so the results cannot be generalized to other samples. Furthermore, the treatment of anxiety was restricted to females, whereas the treatment of depression was restricted to males. Additionally, the selection of females and males as samples for the study was based on their pre-measurement of anxiety and depression, which serve as self-report measures.
The authors seem to be unconcerned about the fact that the 2 interventions (verum and control) were clearly distinguishable and their patients thus were not blinded (and neither were the evaluators). This obviously means that the observed effect might have nothing at all to do with the Islamic-Based Intervention but could be entirely due to expectation and persuasion.
Why might the authors not even bother to discuss such an obvious possibility?
A look at their affiliations might provide the answer:
- 1Academy of Islamic Studies, University of Malaya, 50603, Kuala Lumpur, Malaysia. [email protected].
- 2Academy of Islamic Studies, University of Malaya, 50603, Kuala Lumpur, Malaysia.
- 3Faculty of Education, Universiti Teknologi Malaysia, Johor, Malaysia.
- 4Faculty of Education, University of Malaya, 50603, Kuala Lumpur, Malaysia.
- 5Islamic Banking and Finance, International Islamic University Malaysia, Selangor, Malaysia.
- 6Department of Hadith and Associated Sciences, Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia.
I rarely follow up announcements of new studies of so-called alternative medicine (SCAM). But this one is different. It was so spectacular that almost precisely two years ago I reported about it. Here is what I wrote:
Dr. Dhanunjaya Lakkireddy, a cardiologist at the Kansas City Heart Rhythm Institute in the US, has started a trial of prayer for corona-virus infection. The study will involve 1000 patients with COVID-19 infections severe enough to require intensive care. The four-month study will investigate “the role of remote intercessory multi-denominational prayer on clinical outcomes in COVID-19 patients,” according to a description provided to the National Institutes of Health.
Inclusion Criteria:
- Male or female greater than 18 years of age
- Confirmed positive for COVID-19
- Patient admitted to Intensive Care Unit
Exclusion Criteria:
- Patients admitted to ICU for diagnosis that is not COVID-19 positive
(Not giving informed consent is not listed as an exclusion criterion!)
Half of the patients, randomly chosen, will receive a “universal” prayer offered in five denominational forms, via:
- Buddhism,
- Christianity,
- Hinduism,
- Islam,
- Judaism.
The other 500 patients in the control group will not be prayed for by the prayer group. All the patients will receive the standard care prescribed by their medical providers. “We all believe in science, and we also believe in faith,” Lakkireddy claims. “If there is a supernatural power, which a lot of us believe, would that power of prayer and divine intervention change the outcomes in a concerted fashion? That was our question.”
The outcome measures in the trial are
- the time patients remain on ventilators,
- the number of patients who suffer from organ failure,
- the time patients have to stay in intensive care,
- the mortality rate.
________________________________________
For months, I have now been looking out for the results of this study. It must long be finished now. The results cannot be difficult to analyze. The publication of such a sensational trial should not be a problem. If the findings are positive, even top journals would be keen to publish them. If they are negative, they would still be worth reporting.
So, where are they?
I could not find a trace of them!
Why not?
I was puzzled and became more and more frustrated.
Until I had the obvious idea of looking at the website that reported the above details two years ago. There was the answer to my questions:
“Recruitment Status: Terminated (Due to the low enrollment, this study is closed. Analysis of data is not performed.)”
But how can this be?
What can possibly be the reason for an enrollment that was too low to properly conclude this trial?
- There were certainly enough COVID patients (contrary to what was claimed earlier, the sample size is now given at merely 200).
- Many of these US patients would, of course, be religious and thus welcome some divine intervention.
So, why might such a trial fail? I can only think of two reasons:
- The execution of the trial was sloppy and half-hearted.
- The research question was too daft for participants to consent.
Whatever the reason, I find it sad, possibly even unethical that research funds are being wasted on such nonsense.
PS
The sponsor of this study was the Kansas City Heart Rhythm Institute. The director of the Kansas City Heart Rhythm Institute is Dhanunjaya Lakkireddy, the principal investigator of this trial.
PPS
When the study was first announced in 2020, it received huge publicity. I, therefore, think that the investigators should have had the decency to also publicly announce that they failed to conclude it.
Stress is associated with a multitude of physical and psychological health impairments. To tackle these health disorders, over-the-counter (OTC) products like Neurodoron® are popular since they are considered safe and tolerable. One tablet of this anthroposophic remedy contains the following active ingredients:
- 83.3 mg Aurum metallicum praeparatum trituration (trit.) D10,
- 83.3 mg Kalium phosphoricicum trit. D6,
- 8.3 mg Ferrum-Quarz trit. D2.
Experience reports and first studies indicate that Neurodoron® is efficient in the treatment of stress-associated health symptoms. “To confirm this” (!!!), a non-interventional study (NIS) with pharmacies was conducted.
The NIS was planned to enroll female and male patients who suffered from nervous exhaustion with symptoms caused by acute and/or chronic stress. The main outcome measures were characteristic stress symptoms, stress burden, and perceived stress. Further outcome measures included perceived efficacy and tolerability of the product as assessed by the patients and collection of adverse drug reactions (ADRs). A study duration of about 21 days with a recommended daily dose of 3–4 tablets was set.
In total, 279 patients were enrolled at 74 German pharmacies. The analyzed set (AS) included 272 patients (mean age 44.8 ± 14.4 years, 73.9% female). 175 patients of the AS completed the NIS. During the study, all stress symptoms declined significantly (total score 18.1 vs. 12.1 (of max. 39 points), < 0.0001). Furthermore, a reduction of stress burden (relative difference in stress burden, VAS = −29.1%, < 0.0001) was observed. For most patients, perceived stress was reduced at the study end (PSQ total score decreased in 70.9% of the patients). 75.9% of the study population rated the product efficacy as “good” or “very good” and 96.6% rated its tolerability as “good” or “very good.” One uncritical ADR was reported.
The authors concluded that this study adds information on the beneficial effects of Neurodoron® in self-medication. The results from this NIS showed a marked reduction in stress burden and perceived stress, along with an excellent safety profile of the medicinal product (MP) Neurodoron®. Further trials are required to confirm these results.
I beg to differ!
The study had no control group and therefore one cannot possibly attribute any of the observed changes to the anthroposophic remedy. They are more likely to be due to:
- the natural history of the condition,
- regression towards the mean,
- a placebo effects,
- other treatments administered during the trial period.
Sadly, the authors discuss none of these possibilities in their paper.
In view of this, I am tempted to rephrase their conclusions as follows:
This study adds no valuable information on the effects of Neurodoron® in self-medication. The results from this NIS showed what utter nonsense the Weleda marketing team is capable of producing in an attempt to boost sales.
PS
These declarations of the 4 study authors and the sponsorship are revealing, I thought:
RH and CS are employees of Weleda AG, Germany. JH and KS work for daacro GmbH & Co. KG, a clinical research organization, Germany. The authors declare that there are no conflicts of interest.
This study was financed by the pharmaceutical company Weleda AG, Arlesheim, the employer of RH and CS. Weleda commissioned the CRO daacro for their contribution to the manuscript.
On 27 January 2022, I conducted a very simple Medline search using the search term ‘Chinese Herbal Medicine, Review, 2022’. Its results were remarkable; here are the 30 reviews I found:
- Zhu, S. J., Wang, R. T., Yu, Z. Y., Zheng, R. X., Liang, C. H., Zheng, Y. Y., Fang, M., Han, M., & Liu, J. P. (2022). Chinese herbal medicine for myasthenia gravis: A systematic review and meta-analysis of randomized clinical trials. Integrative medicine research, 11(2), 100806.
- Lu, J., Li, W., Gao, T., Wang, S., Fu, C., & Wang, S. (2022). The association study of chemical compositions and their pharmacological effects of Cyperi Rhizoma (Xiangfu), a potential traditional Chinese medicine for treating depression. Journal of ethnopharmacology, 287, 114962.
- Su, F., Sun, Y., Zhu, W., Bai, C., Zhang, W., Luo, Y., Yang, B., Kuang, H., & Wang, Q. (2022). A comprehensive review of research progress on the genus Arisaema: Botany, uses, phytochemistry, pharmacology, toxicity and pharmacokinetics. Journal of ethnopharmacology, 285, 114798.
- Nanjala, C., Ren, J., Mutie, F. M., Waswa, E. N., Mutinda, E. S., Odago, W. O., Mutungi, M. M., & Hu, G. W. (2022). Ethnobotany, phytochemistry, pharmacology, and conservation of the genus Calanthe R. Br. (Orchidaceae). Journal of ethnopharmacology, 285, 114822.
- Li, M., Jiang, H., Hao, Y., Du, K., Du, H., Ma, C., Tu, H., & He, Y. (2022). A systematic review on botany, processing, application, phytochemistry and pharmacological action of Radix Rehmnniae. Journal of ethnopharmacology, 285, 114820.
- Mutinda, E. S., Mkala, E. M., Nanjala, C., Waswa, E. N., Odago, W. O., Kimutai, F., Tian, J., Gichua, M. K., Gituru, R. W., & Hu, G. W. (2022). Traditional medicinal uses, pharmacology, phytochemistry, and distribution of the Genus Fagaropsis (Rutaceae). Journal of ethnopharmacology, 284, 114781.
- Xu, Y., Liu, J., Zeng, Y., Jin, S., Liu, W., Li, Z., Qin, X., & Bai, Y. (2022). Traditional uses, phytochemistry, pharmacology, toxicity and quality control of medicinal genus Aralia: A review. Journal of ethnopharmacology, 284, 114671.
- Peng, Y., Chen, Z., Li, Y., Lu, Q., Li, H., Han, Y., Sun, D., & Li, X. (2022). Combined therapy of Xiaoer Feire Kechuan oral liquid and azithromycin for mycoplasma Pneumoniae pneumonia in children: A systematic review & meta-analysis. Phytomedicine : international journal of phytotherapy and phytopharmacology, 96, 153899.
- Xu, W., Li, B., Xu, M., Yang, T., & Hao, X. (2022). Traditional Chinese medicine for precancerous lesions of gastric cancer: A review. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 146, 112542.
- Wang, Y., Greenhalgh, T., Wardle, J., & Oxford TCM Rapid Review Team (2022). Chinese herbal medicine (“3 medicines and 3 formulations”) for COVID-19: rapid systematic review and meta-analysis. Journal of evaluation in clinical practice, 28(1), 13–32.
- Chen, X., Lei, Z., Cao, J., Zhang, W., Wu, R., Cao, F., Guo, Q., & Wang, J. (2022). Traditional uses, phytochemistry, pharmacology and current uses of underutilized Xanthoceras sorbifolium bunge: A review. Journal of ethnopharmacology, 283, 114747.
- Liu, X., Li, Y., Bai, N., Yu, C., Xiao, Y., Li, C., & Liu, Z. (2022). Updated evidence of Dengzhan Shengmai capsule against ischemic stroke: A systematic review and meta-analysis. Journal of ethnopharmacology, 283, 114675.
- Chen, J., Zhu, Z., Gao, T., Chen, Y., Yang, Q., Fu, C., Zhu, Y., Wang, F., & Liao, W. (2022). Isatidis Radix and Isatidis Folium: A systematic review on ethnopharmacology, phytochemistry and pharmacology. Journal of ethnopharmacology, 283, 114648.
- Tian, J., Shasha, Q., Han, J., Meng, J., & Liang, A. (2022). A review of the ethnopharmacology, phytochemistry, pharmacology and toxicology of Fructus Gardeniae (Zhi-zi). Journal of ethnopharmacology, 114984. Advance online publication.
- Wong, A. R., Yang, A., Li, M., Hung, A., Gill, H., & Lenon, G. B. (2022). The Effects and Safety of Chinese Herbal Medicine on Blood Lipid Profiles in Placebo-Controlled Weight-Loss Trials: A Systematic Review and Meta-Analysis. Evidence-based complementary and alternative medicine : eCAM, 2022, 1368576.
- Lu, C., Ke, L., Li, J., Wu, S., Feng, L., Wang, Y., Mentis, A., Xu, P., Zhao, X., & Yang, K. (2022). Chinese Medicine as an Adjunctive Treatment for Gastric Cancer: Methodological Investigation of meta-Analyses and Evidence Map. Frontiers in pharmacology, 12, 797753.
- Niu, L., Xiao, L., Zhang, X., Liu, X., Liu, X., Huang, X., & Zhang, M. (2022). Comparative Efficacy of Chinese Herbal Injections for Treating Severe Pneumonia: A Systematic Review and Bayesian Network Meta-Analysis of Randomized Controlled Trials. Frontiers in pharmacology, 12, 743486.
- Zhang, L., Huang, J., Zhang, D., Lei, X., Ma, Y., Cao, Y., & Chang, J. (2022). Targeting Reactive Oxygen Species in Atherosclerosis via Chinese Herbal Medicines. Oxidative medicine and cellular longevity, 2022, 1852330.
- Zhou, X., Guo, Y., Yang, K., Liu, P., & Wang, J. (2022). The signaling pathways of traditional Chinese medicine in promoting diabetic wound healing. Journal of ethnopharmacology, 282, 114662.
- Yang, M., Shen, C., Zhu, S. J., Zhang, Y., Jiang, H. L., Bao, Y. D., Yang, G. Y., & Liu, J. P. (2022). Chinese patent medicine Aidi injection for cancer care: An overview of systematic reviews and meta-analyses. Journal of ethnopharmacology, 282, 114656.
- Liu, H., & Wang, C. (2022). The genus Asarum: A review on phytochemistry, ethnopharmacology, toxicology and pharmacokinetics. Journal of ethnopharmacology, 282, 114642.
- Lin, Z., Zheng, J., Chen, M., Chen, J., & Lin, J. (2022). The Efficacy and Safety of Chinese Herbal Medicine in the Treatment of Knee Osteoarthritis: An Updated Systematic Review and Meta-Analysis of 56 Randomized Controlled Trials. Oxidative medicine and cellular longevity, 2022, 6887988.
- Yu, R., Zhang, S., Zhao, D., & Yuan, Z. (2022). A systematic review of outcomes in COVID-19 patients treated with western medicine in combination with traditional Chinese medicine versus western medicine alone. Expert reviews in molecular medicine, 24, e5.
- Mo, X., Guo, D., Jiang, Y., Chen, P., & Huang, L. (2022). Isolation, structures and bioactivities of the polysaccharides from Radix Hedysari: A review. International journal of biological macromolecules, 199, 212–222.
- Yang, L., Chen, X., Li, C., Xu, P., Mao, W., Liang, X., Zuo, Q., Ma, W., Guo, X., & Bao, K. (2022). Real-World Effects of Chinese Herbal Medicine for Idiopathic Membranous Nephropathy (REACH-MN): Protocol of a Registry-Based Cohort Study. Frontiers in pharmacology, 12, 760482.
- Zhang, R., Zhang, Q., Zhu, S., Liu, B., Liu, F., & Xu, Y. (2022). Mulberry leaf (Morus alba L.): A review of its potential influences in mechanisms of action on metabolic diseases. Pharmacological research, 175, 106029.
- Yuan, J. Y., Tong, Z. Y., Dong, Y. C., Zhao, J. Y., & Shang, Y. (2022). Research progress on icariin, a traditional Chinese medicine extract, in the treatment of asthma. Allergologia et immunopathologia, 50(1), 9–16.
- Zeng, B., Wei, A., Zhou, Q., Yuan, M., Lei, K., Liu, Y., Song, J., Guo, L., & Ye, Q. (2022). Andrographolide: A review of its pharmacology, pharmacokinetics, toxicity and clinical trials and pharmaceutical researches. Phytotherapy research : PTR, 36(1), 336–364.
- Zhang, L., Xie, Q., & Li, X. (2022). Esculetin: A review of its pharmacology and pharmacokinetics. Phytotherapy research : PTR, 36(1), 279–298.
- Wang, D. C., Yu, M., Xie, W. X., Huang, L. Y., Wei, J., & Lei, Y. H. (2022). Meta-analysis on the effect of combining Lianhua Qingwen with Western medicine to treat coronavirus disease 2019. Journal of integrative medicine, 20(1), 26–33. https://doi.org/10.1016/j.joim.2021.10.005
The amount of reviews alone is remarkable, I think: more than one review per day! Apart from their multitude, the reviews are noteworthy for other reasons as well.
- Their vast majority arrived at positive or at least encouraging conclusions.
- Most of the primary studies are from China (and we have often discussed how unreliable these trials are).
- Many of the primary studies are not accessible.
- Those that are accessible tend to be of lamentable quality.
I fear that all this is truly dangerous. The medical literature is being swamped with reviews of Chinese herbal medicine and other TCM modalities. Collectively they give the impression that these treatments are supported by sound evidence. Yet, the exact opposite is the case.
The process that is happening in front of our very eyes is akin to that of money laundering. Unreliable and often fraudulent data is being white-washed and presented to us as evidence.
The result:
WE ARE BEING SYSTEMATICALLY MISLED!
Auriculotherapy (or ear acupuncture) is the use of electrical, mechanical, or other stimuli at specific points on the outer ear for therapeutic purposes. It was invented by the French neurologist Paul Nogier (1908–1996) who published his “Treatise of Auriculotherapy” in 1961. Auriculotherapy is based on the idea that the human outer ear is an area that reflects the entire body. Proponents of auriculotherapy refer to maps where our inner organs and body parts are depicted on the outer ear. These maps are not in line with our knowledge of anatomy and physiology. Auriculotherapy thus lacks plausibility.
This single-blind randomized, placebo-controlled study aimed to investigate the effect of auriculotherapy on the intensity of Premenstrual Syndrome (PMS) symptoms.
Ninety-one women were randomly assigned to
- Auriculotherapy (AG),
- Placebo (PG),
- Control (CG) groups.
The intervention was 8 weeks long, done once per week. At each session in AG the microneedles were placed in seven points related to PMS symptoms (Anxiety; Endocrine; Muscle relaxation; Analgesia; Kidney; Shen Men; and Sympathetic). At PG the microneedles also were placed in seven points but unrelated to PMS symptoms (Tonsils; Vocal cords; Teeth; Eyes; Allergy; Mouth; and External nose). The women allocate in the CG received o intervention during the evaluation period.
Assessments of PMS symptoms (Premenstrual Syndrome Screening Tool), musculoskeletal pain (Nordic Musculoskeletal Questionnaire), anxiety (Beck Anxiety Inventory), and quality of life (WHOQOL-Bref) were done at baseline, before the 5th session, after program completion, and a month follow-up.
The AG and PG showed significantly lower scores of PMS symptoms, musculoskeletal pain, and anxiety. On the quality of life and follow-up analysis, the significance was observed only in PG.
The authors concluded that auriculotherapy can be used as adjunctive therapy to reduce the physical and mood PMS symptoms.
If I understand it correctly (the paper is unclear), verum and placebo were both better than no intervention but showed no significant differences when compared to each other. This is strong evidence that auriculotherapy is, in fact, a placebo. To make matters worse, in the follow-up analysis placebo seems to be superior to auriculotherapy.
Another issue might be adverse effects. Microneedle implants can cause severe complications. Thus it is mandatory to monitor adverse effects in clinical trials. This does not seem to have happened in this case.
The mind boggles!
How on earth could the authors conclude that auriculotherapy can be used as adjunctive therapy to reduce the physical and mood PMS symptoms.
The answer: a case of scientific misconduct?
This systematic review examined the efficacy of acupressure on depression. Literature searches were performed on PubMed, PsycINFO, Scopus, Embase, MEDLINE, and China National Knowledge (CNKI). Randomized clinical trials (RCTs) or single-group trials in which acupressure was compared with various control methods or baseline (i.e. no treatment) in people with depression were included. Data were synthesized using a random-effects or a fixed-effects model to analyze the impacts of acupressure treatment on depression and anxiety in people with depression. The primary outcome measures were depression symptoms quantified by various means. Subgroups were created, and meta-regression analyses were performed to explore which factors are relevant to the greater or lesser effects of treating symptoms. 
A total of 14 RCTs (1439 participants) were identified. Analysis of the between-group showed that acupressure was effective in reducing depression [Standardized mean differences (SMDs) = -0.58, 95%CI: -0.85 to -0.32, P < 0.0001] and anxiety (SMD = -0.67, 95%CI: -0.99 to -0.36, P < 0.0001) in participants with mild-to-moderate primary and secondary depression. Subgroup analyses suggested that acupressure significantly reduced depressive symptoms compared with different controlled conditions and in participants with different ages, clinical conditions, and duration of intervention. Adverse events, including hypotension, dizziness, palpitation, and headache, were reported in only one study.
The authors concluded that the evidence of acupressure for mild-to-moderate depressive symptoms was significant. Importantly, the findings should be interpreted with caution due to study limitations. Future research with a well-designed mixed method is required to consolidate the conclusion and provide an in-depth understanding of potential mechanisms underlying the effects.
I think that more than caution is warranted when interpreting these data. In fact, it would have been surprising if the meta-analyses had NOT generated an overall positive result. This is because in several studies there was no attempt to control for the extra attention or the placebo effect of administering acupressure. In most of the trials where this had been taken care of (i.e. patient-blinded, sham-controlled studies), there were no checks for the success of blinding. Thus it is possible, even likely that many patients correctly guessed what treatment they received. In turn, this means that the outcomes of these trials were also largely due to placebo effects.
Overall, this paper is therefore a prime example of a biased review of biased primary studies. The phenomenon can be aptly described by the slogan:
RUBBISH IN, RUBBISH OUT!



