scientific misconduct
Ginkgo biloba is a well-researched herbal medicine which has shown promise for a number of indications. But does this include coronary heart disease?
The aim of this systematic review was to provide information about the effectiveness and safety of Ginkgo Leaf Extract and Dipyridamole Injection (GD) as one adjuvant therapy for treating angina pectoris (AP) and to evaluate the relevant randomized controlled trials (RCTs) with meta-analysis. (Ginkgo Leaf Extract and Dipyridamole Injection is a Chinese compound preparation, which consists of ginkgo flavone glycosides (24%), terpene lactones (ginkgolide about 13%, ginkgolide about 2.9%) and dipyridamole.)
RCTs concerning AP treated by GD were searched and the Cochrane Risk Assessment Tool was adopted to assess the methodological quality of the RCTs. A total of 41 RCTs involving 4,462 patients were included in the meta-analysis. The results indicated that the combined use of GD and Western medicine (WM) against AP was associated with a higher total effective rate [risk ratio (RR)=1.25, 95% confidence interval (CI): 1.21–1.29, P<0.01], total effective rate of electrocardiogram (RR=1.29, 95% CI: 1.21–1.36, P<0.01). Additional, GD combined with WM could decrease the level of plasma viscosity [mean difference (MD)=–0.56, 95% CI:–0,81 to–0.30, P<0.01], fibrinogen [MD=–1.02, 95% CI:–1.50 to–0.54, P<0.01], whole blood low shear viscosity [MD=–2.27, 95% CI:–3.04 to–1.49, P<0.01], and whole blood high shear viscosity (MD=–0.90, 95% CI: 1.37 to–0.44, P<0.01).
The authors concluded that comparing with receiving WM only, the combine use of GD and WM was associated with a better curative effect for patients with AP. Nevertheless, limited by the methodological quality of included RCTs more large-sample, multi-center RCTs were needed to confirm our findings and provide further evidence for the clinical utility of GD.
If one reads this conclusion, one might be tempted to use GD to cure AP. I would, however, strongly warn everyone from doing so. There are many reasons for my caution:
- All the 41 RCTs originate from China, and we have repeatedly discussed that Chinese TCM trials are highly unreliable.
- The methodological quality of the primary RCTs was, according to the review authors ‘moderate’. This is not true; it was, in fact, lousy.
- Dipyridamole is not indicated in angina pectoris.
- To the best of my knowledge, there is no good evidence from outside China to suggest that Ginkgo biloba is effective for angina pectoris.
- Angina pectoris is caused by coronary artery disease (a narrowing of one or more coronary arteries due to atherosclerosis), and it seems implausible that this condition can be ‘cured’ with any medication.
So, what we have here is yet another nonsensical paper, published in a dubious journal, employing evidently irresponsible reviewers, run by evidently irresponsible editors, hosted by a seemingly reputable publisher (Springer). This is reminiscent of my previous post (and many posts before). Alarmingly, it is also what I encounter on a daily basis when scanning the new publications in my field.
The effects of this incessant stream of nonsense can only have one of two effects:
- People take this ‘evidence’ seriously. In this case, many patients might pay with their lives for this collective incompetence.
- People conclude that alt med research cannot be taken seriously. In this case, we are unlikely to ever see anything useful emerging from it.
Either way, the result will be profoundly negative!
It is high time to stop this idiocy; but how?
I wish, I knew the answer.
After 25 years of full-time research into alternative medicine, I thought that I have seen it all. But I was wrong! Here is an article that surpasses every irresponsible stupidity I can remember. It is entitled ‘Ginger is the monumentally superior alternative to chemotherapy‘:
Let’s say that your doctor has given you a cancer diagnosis. Let’s revisit animal wisdom. If a squirrel was looking over a tasty morsel of ginger on one side, or a vial full of Mehotrexate, Danorubicin or Tioguanine on the other, what would that intelligent squirrel choose? The answer is obvious. And it’s the right answer, because ginger roots, after being dried and cooked, manifest an ingredient called 6-shogaol.
This naturally occurring element is up to 10,000 times more effective at killing cancer cells than those vials of destructive drugs, reports David Guiterrez from Natural News, who states that “researchers found that 6-shogaol is active against cancer stem cells at concentrations that are harmless to healthy cells. This is dramatically different from conventional chemotherapy, which has serious side effects largely because it kills healthy as well as cancerous cells.”
END OF QUOTE
As David Guiterrez from Natural News might not be the most reliable of sources, I did a bit of searching for evidence. This is what I found:
A study examining the efficacy of ginger, as an adjuvant drug to standard antiemetic therapy, in ameliorating acute and delayed CINV in patients with lung cancer receiving cisplatin-based regimens. It concluded that as an adjuvant drug to standard antiemetic therapy, ginger had no additional efficacy in ameliorating CINV in patients with lung cancer receiving cisplatin-based regimens.
A randomized, double-blind, placebo-controlled, multicenter study in patients planned to receive ≥2 chemotherapy cycles with high dose (>50 mg/m2) cisplatin. Patients received ginger 160 mg/day (with standardized dose of bioactive compounds) or placebo in addition to the standard antiemetic prophylaxis for CINV, starting from the day after cisplatin administration. The authors found that in patients treated with high-dose cisplatin, the daily addition of ginger, even if safe, did not result in a protective effect on CINV.
Yes, there are also a few trials to suggest that ginger is effective for reducing nausea and vomiting after chemotherapy, but by and large they are older and less rigorous. And anyway, this is besides the point. The question here is not whether there is good evidence to show that ginger is helpful against chemo-induced nausea; the question is whether Ginger is clinically effective in ‘killing cancer cells’. And the answer is an emphatic
NO!!!
And this means the above-quoted article irresponsible, unethical, perhaps even criminal to the extreme. I shudder to think how many cancer patients have read it and consequently given up their conventional treatments opting for Ginger instead.
This systematic review was aimed at evaluating the effects of acupuncture on the quality of life of migraineurs. Only randomized controlled trials that were published in Chinese and English were included. In total, 62 trials were included for the final analysis; 50 trials were from China, 3 from Brazil, 3 from Germany, 2 from Italy and the rest came from Iran, Israel, Australia and Sweden.
Acupuncture resulted in lower Visual Analog Scale scores than medication at 1 month after treatment and 1-3 months after treatment. Compared with sham acupuncture, acupuncture resulted in lower Visual Analog Scale scores at 1 month after treatment.
The authors concluded that acupuncture exhibits certain efficacy both in the treatment and prevention of migraines, which is superior to no treatment, sham acupuncture and medication. Further, acupuncture enhanced the quality of life more than did medication.
The authors comment in the discussion section that the overall quality of the evidence for most outcomes was of low to moderate quality. Reasons for diminished quality consist of the following: no mentioned or inadequate allocation concealment, great probability of reporting bias, study heterogeneity, sub-standard sample size, and dropout without analysis.
Further worrisome deficits are that only 14 of the 62 studies reported adverse effects (this means that 48 RCTs violated research ethics!) and that there was a high level of publication bias indicating that negative studies had remained unpublished. However, the most serious concern is the fact that 50 of the 62 trials originated from China, in my view. As I have often pointed out, such studies have to be categorised as highly unreliable.
In view of this multitude of serious problems, I feel that the conclusions of this review must be re-formulated:
Despite the fact that many RCTs have been published, the effect of acupuncture on the quality of life of migraineurs remains unproven.
I only recently came across this review; it was published a few years ago but is still highly relevant. It summarizes the evidence of controlled clinical studies of TCM for cancer.
The authors searched all the controlled clinical studies of TCM therapies for all kinds of cancers published in Chinese in four main Chinese electronic databases from their inception to November 2011. They found a total of 2964 reports (involving 253,434 cancer patients) including 2385 randomized controlled trials and 579 non-randomized controlled studies.
The top seven cancer types treated were lung cancer, liver cancer, stomach cancer, breast cancer, esophagus cancer, colorectal cancer and nasopharyngeal cancer by both study numbers and case numbers. The majority of studies (72%) applied TCM therapy combined with conventional treatment, whilst fewer (28%) applied only TCM therapy in the experimental groups. Herbal medicine was the most frequently applied TCM therapy (2677 studies, 90.32%). The most frequently reported outcome was clinical symptom improvement (1667 studies, 56.24%) followed by biomarker indices (1270 studies, 42.85%), quality of life (1129 studies, 38.09%), chemo/radiotherapy induced side effects (1094 studies, 36.91%), tumour size (869 studies, 29.32%) and safety (547 studies, 18.45%).
The authors concluded that data from controlled clinical studies of TCM therapies in cancer treatment is substantial, and different therapies are applied either as monotherapy or in combination with conventional medicine. Reporting of controlled clinical studies should be improved based on the CONSORT and TREND Statements in future. Further studies should address the most frequently used TCM therapy for common cancers and outcome measures should address survival, relapse/metastasis and quality of life.
This paper is important, in my view, predominantly because it exemplifies the problem with TCM research from China and with uncritical reviews on this subject. If a cancer patient, who does not know the background, reads this paper, (s)he might think that TCM is worth trying. This conclusion could easily shorten his/her life.
The often-shown fact is that TCM studies from China are not reliable. They are almost invariably positive, their methodological quality is low, and they are frequently based on fabricated data. In my view, it is irresponsible to publish a review that omits discussing these facts in detail and issuing a stark warning.
TCM FOR CANCER IS A VERY BAD CHOICE!
In the latest issue of ‘Simile’ (the Faculty of Homeopathy‘s newsletter), the following short article with the above title has been published. I took the liberty of copying it for you:
Members of the Faculty of Homeopathy practising in the UK have the opportunity to take part in a trial of a new homeopathic remedy for treating infant colic. An American manufacturer of homeopathic remedies has made a registration application for the new remedy to the MHRA (Medicines and Healthcare products Regulatory Agency) under the UK “National Rules” scheme. As part of its application the manufacturer is seeking at least two homeopathic doctors who would be willing to trial the product for about a year, then write a short report about using the remedy and its clinical results. If you would like to take part in the trial, further details can be obtained from …
END OF QUOTE
A homeopathic remedy for infant colic?
Yes, indeed!
The British Homeopathic Association and many similar ‘professional’ organisations recommend homeopathy for infant colic: Infantile colic is a common problem in babies, especially up to around sixteen weeks of age. It is characterised by incessant crying, often inconsolable, usually in the evenings and often through the night. Having excluded underlying pathology, the standard advice given by GPs and health visitors is winding technique, Infacol or Gripe Water. These measures are often ineffective but fortunately there are a number of homeopathic medicines that may be effective. In my experience Colocynth is the most successful; alternatives are Carbo Veg, Chamomilla and Nux vomica.
SO, IT MUST BE GOOD!
But hold on, I cannot find a single clinical trial to suggest that homeopathy is effective for infant colic.
Ahhhhhhhhhhhhhhhhhhh, I see, that’s why they now want to conduct a trial!
They want to do the right thing and do some science to see whether their claims are supported by evidence.
How very laudable!
After all, the members of the Faculty of Homeopathy are doctors; they have certain ethical standards!
After all, the Faculty of Homeopathy aims to provide a high level of service to members and members of the public at all times.
Judging from the short text about the ‘homeopathy for infant colic trial’, it will involve a few (at least two) homeopaths prescribing the homeopathic remedy to patients and then writing a report. These reports will unanimously state that, after the remedy had been administered, the symptoms improved considerably. (I know this because they always do improve – with or without treatment.)
These reports will then be put together – perhaps we should call this a meta-analysis? – and the overall finding will be nice, positive and helpful for the American company.
And now, we all understand what homeopaths, more precisely the Faculty of Homeopathy, consider to be evidence.
Acupuncture is a branch of alternative medicine where pseudo-science abounds. Here is yet another example of this deplorable phenomenon.
This study was conducted to evaluate the efficacy of acupuncture in the management of primary dysmenorrhea.
Sixty females aged 17-23 years were randomly assigned to either a study group or a control group.
- The study group received acupuncture for the duration of 20 minutes/day, for 15 days/month, for the period of 90 days.
- The control group did not receive acupuncture for the same period.
Both groups were assessed on day 1; day 30 and day 60; and day 90. The results showed a significant reduction in all the variables such as the visual analogue scale score for pain, menstrual cramps, headache, dizziness, diarrhoea, faint, mood changes, tiredness, nausea, and vomiting in the study group compared with those in the control group.
The authors concluded that acupuncture could be considered as an effective treatment modality for the management of primary dysmenorrhea.
These findings contradict those of a recent Cochrane review (authored by known acupuncture-proponents) which included 42 RCTs and concluded that there is insufficient evidence to demonstrate whether or not acupuncture or acupressure are effective in treating primary dysmenorrhoea, and for most comparisons no data were available on adverse events. The quality of the evidence was low or very low for all comparisons. The main limitations were risk of bias, poor reporting, inconsistency and risk of publication bias.
The question that I ask myself is this: why do researchers bother to conduct studies that contribute NOTHING to our knowledge and progress? The new study had a no-treatment control group which means it cannot control for the effects of placebo, the extra attention, social desirability etc. In view of the fact that already 42 poor quality trials exist, it is not just useless to add a 43rd but, in my view, it is scandalous! A 43rd useless trial:
- tells us nothing of value;
- misleads the public;
- pollutes the medical literature;
- is a waste of resources;
- undermines the trust in clinical research;
- is deeply unethical.
It is high time to stop such redundant, foolish, wasteful and unethical pseudo-science.
Homeopathy for depression? A previous review concluded that the evidence for the effectiveness of homeopathy in depression is limited due to lack of clinical trials of high quality. But that was 13 years ago. Perhaps the evidence has changed?
A new review aimed to assess the efficacy, effectiveness and safety of homeopathy in depression. Eighteen studies assessing homeopathy in depression were included. Two double-blind placebo-controlled trials of homeopathic medicinal products (HMPs) for depression were assessed.
- The first trial (N = 91) with high risk of bias found HMPs were non-inferior to fluoxetine at 4 and 8 weeks.
- The second trial (N = 133), with low risk of bias, found HMPs was comparable to fluoxetine and superior to placebo at 6 weeks.
The remaining research had unclear/high risk of bias. A non-placebo-controlled RCT found standardised treatment by homeopaths comparable to fluvoxamine; a cohort study of patients receiving treatment provided by GPs practising homeopathy reported significantly lower consumption of psychotropic drugs and improved depression; and patient-reported outcomes showed at least moderate improvement in 10 of 12 uncontrolled studies. Fourteen trials provided safety data. All adverse events were mild or moderate, and transient. No evidence suggested treatment was unsafe.
The authors concluded that limited evidence from two placebo-controlled double-blinded trials suggests HMPs might be comparable to antidepressants and superior to placebo in depression, and patients treated by homeopaths report improvement in depression. Overall, the evidence gives a potentially promising risk benefit ratio. There is a need for additional high quality studies.
I beg to differ!
What these data really show amounts to far less than the authors imply:
- The two ‘double-blind’ trials are next to meaningless. As equivalence studies they were far too small to produce meaningful results. Any decent review should discuss this fact in full detail. Moreover, these studies cannot have been double-blind, because the typical adverse-effects of anti-depressants would have ‘de-blinded’ the trial participants. Therefore, these results are almost certainly false-positive.
- The other studies are even less rigorous and therefore do also not allow positive conclusions.
This review was authored by known proponents of homeopathy. It is, in my view, an exercise in promotion rather than a piece of research. I very much doubt that a decent journal with a responsible peer-review system would have ever published such a biased paper – it had to appear in the infamous EUROPEAN JOURNAL OF INTEGRATIVE MEDICINE.
So what?
Who cares? No harm done!
Again, I beg to differ.
Why?
The conclusion that homeopathy has a ‘promising risk/benefit profile’ is frightfully dangerous and irresponsible. If seriously depressed patients follow it, many lives might be lost.
Yet again, we see that poor research has the potential to kill vulnerable individuals.
On Twitter and elsewhere, homeopaths have been celebrating: FINALLY A PROOF OF HOMEOPATHY HAS BEEN PUBLISHED IN A TOP SCIENCE JOURNAL!!!
Here is just one example:
#homeopathy under threat because of lack of peer reviewed studies in respectable journals? Think again. Study published in the most prestigious journal Nature shows efficacy of rhus tox in pain control in rats.
But what exactly does this study show (btw, it was not published in ‘Nature’)?
The authors of the paper in question evaluated antinociceptive efficacy of Rhus Tox in the neuropathic pain and delineated its underlying mechanism. Initially, in-vitro assay using LPS-mediated ROS-induced U-87 glioblastoma cells was performed to study the effect of Rhus Tox on reactive oxygen species (ROS), anti-oxidant status and cytokine profile. Rhus Tox decreased oxidative stress and cytokine release with restoration of anti-oxidant systems. Chronic treatment with Rhus Tox ultra dilutions for 14 days ameliorated neuropathic pain revealed as inhibition of cold, warm and mechanical allodynia along with improved motor nerve conduction velocity (MNCV) in constricted nerve. Rhus Tox decreased the oxidative and nitrosative stress by reducing malondialdehyde (MDA) and nitric oxide (NO) content, respectively along with up regulated glutathione (GSH), superoxide dismutase (SOD) and catalase activity in sciatic nerve of rats. Notably, Rhus Tox treatment caused significant reductions in the levels of tumor necrosis factor (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β) as compared with CCI-control group. Protective effect of Rhus Tox against CCI-induced sciatic nerve injury in histopathology study was exhibited through maintenance of normal nerve architecture and inhibition of inflammatory changes. Overall, neuroprotective effect of Rhus Tox in CCI-induced neuropathic pain suggests the involvement of anti-oxidative and anti-inflammatory mechanisms.
END OF QUOTE
I am utterly under-whelmed by in-vitro experiments (which are prone to artefacts) and animal studies (especially those with a sample size of 8!) of homeopathy. I think they have very little relevance to the question whether homeopathy works.
But there is more, much more!
It has been pointed out that there are several oddities in this paper which are highly suspicious of scientific misconduct or fraud. It has been noted that the study used duplicated data figures that claimed to show different experimental results, inconsistently reported data and results for various treatment dilutions in the text and figures, contained suspiciously identical data points throughout a series of figures that were reported to represent different experimental results, and hinged on subjective, non-blinded data from a pain experiment involving just eight rats.
Lastly, others pointed out that even if the data is somehow accurate, the experiment is unconvincing. The fast timing differences of paw withdraw is subjective. It’s also prone to bias because the researchers were not blinded to the rats’ treatments (meaning they could have known which animals were given the control drug or the homeopathic dilution). Moreover, eight animals in each group is not a large enough number from which to draw firm conclusions, they argue.
As one consequence of these suspicions, the journal has recently added the following footnote to the publication:
10/1/2018 Editors’ Note: Readers are alerted that the conclusions of this paper are subject to criticisms that are being considered by the editors. Appropriate editorial action will be taken once this matter is resolved.
WATCH THIS SPACE!
Bee venom acupuncture is a form of acupuncture in which bee venom is applied to the tips of acupuncture needles, stingers are extracted from bees, or bees are held with an instrument exposing the stinger, and applied to acupoints on the skin.
Bee venom consisting of multiple anti-inflammatory compounds such as melittin, adolapin, apamin. Other substances such as phospholipase A2 can be anti-inflammatory in low concentrations and pro-inflammatory in others. However, bee venom also contains proinflammatory substances, melittin, mast cell degranulation peptide 401, and histamine.
Bee venom acupuncture has been used to treat a number of conditions such as lumbar disc disease, osteoarthritis of the knee, rheumatoid arthritis, adhesive capsulitis, lateral epicondylitis, peripheral neuropathies, stroke and Parkinson’s Disease. The quality of these studies tends to be so poor that any verdict on the effectiveness of bee venom acupuncture would be premature.
A new clinical trial of bee-venom acupuncture for rheumatoid arthritis (RA) might change this situation. A total of 120 cases of RA patients were randomized into bee-sting acupuncture group (treatment) and western medicine group (control). The patients of the control group were treated by oral administration of Methotrexate (10 mg, once a week) and Celecoxlb (0.2 g, once a day). Those of the treatment group received 5 to 15 bee stings of Ashi-points or acupoints according to different conditions and corporeity, and with the bee-sting retained for about 5 min every time, once every other day. The treatment lasted for 8 weeks. The therapeutic effect was assessed by examining:
- symptoms and signs of the affected joints as morning stiffness duration,
- swollen/tender joint counts (indexes),
- handgrip strength,
- 15 m-walking time,
- visual analogue scale (VAS),
- Disease Activity Score including a 28-joint count (DAS 28),
- rheumatoid factor (RF),
- erythrocyte sedimentation rate (ESR),
- C-reactive protein (CRP),
- anti-cyclic citrullinated peptide antibody (ACCPA).
For assessing the safety of bee-venom acupuncture, the patients’ responses of fever, enlargement of lymph nodes, regional red and swollen, itching, blood and urine tests for routine were examined.
Findings of DAS 28 responses displayed that of the two 60 cases in the control and bee-venom acupuncture groups, 15 and 18 experienced marked improvement, 33 and 32 were effective, 12 and 10 ineffective, with the effective rates being 80% and 83. 33%, respectively. No significant difference was found between the two groups in the effective rate (P>0.05). After the treatment, both groups have witnessed a marked decrease in the levels of morning stiffness duration, arthralgia index, swollen joint count index, joint tenderness index, 15 m walking time, VAS, RF, ESR, CRP and ACCPA, and an obvious increase of handgrip strength relevant to their own levels of pre-treatment in each group (P<0.05). There were no significant differences between the two groups in the abovementioned indexes (P>0.05). The routine blood test, routine urine test, routine stool test, electrocardiogram result, the function of liver and kidney and other security index were within the normal range, without any significant adverse effects found after bee-stinging treatment.
The authors (from the Department of Acupuncture and Moxibustion, Bao’an Hospital of Traditional Chinese Medicine, Shenzhen, China) concluded that bee-venom acupuncture therapy for RA patients is safe and effective, worthy of popularization and application in clinical practice.
Where to start? There is so much – perhaps I just comment on the conclusion:
- Safety cannot be assessed on the basis of such a small sample. Bee venom can cause anaphylaxis, and several deaths have been reported in patients who successfully received the therapy prior to the adverse event. Because there is no adverse-effect monitoring system, the incidence of adverse events is unknown. Stating that it is safe, is therefore a big mistake.
- The trial was a non-superiority study. As such, it needs a much larger sample to be able to make claims about effectiveness.
- From the above two points, it follows that popularization and application in clinical practice would be a stupid exercise.
So, what is left over from this seemingly rigorous RCT?
NOTHING!
(except perhaps a re-affirmation of my often-voiced fear that we must take TCM-studies from China with more than just one pinch of salt)
Evening primrose oil (EPO) is amongst the best-selling herbal remedies of all times. It is marketed in most countries as a dietary supplement. It is being promoted for eczema, rheumatoid arthritis, premenstrual syndrome, breast pain, menopause symptoms, and many other conditions. EPO seems to be a prime example for the fact that, in alternative medicine, the commercial success of a remedy is not necessarily determined by the strength of the evidence but by the intensity and cleverness of the marketing activities.
Evening primrose oil has been extensively tested in clinical trials for a wide range of conditions, including eczema (atopic dermatitis), postmenopausal symptoms, asthma, psoriasis, cellulite, hyperactivity, multiple sclerosis, schizophrenia, obesity, chronic fatigue syndrome, rheumatoid arthritis, and mastalgia. As I have reported previously, these data were burdened with mischief and scientific misconduct, and it is therefore not easy to differentiate between science, pseudoscience and fraud. The results of the more reliable investigations fail to show that it is effective for any condition. A Cochrane review of 2013, for instance, concluded that supplements of evening primrose oil lack effect on eczema; improvement was similar to respective placebos used in trials.
But now, a new study has emerged that casts doubt on this conclusion. The aim of this double-blinded, placebo-controlled RCT is to evaluate the efficacy and safety of EPO in Korean patients with atopic dermatitis (AD).
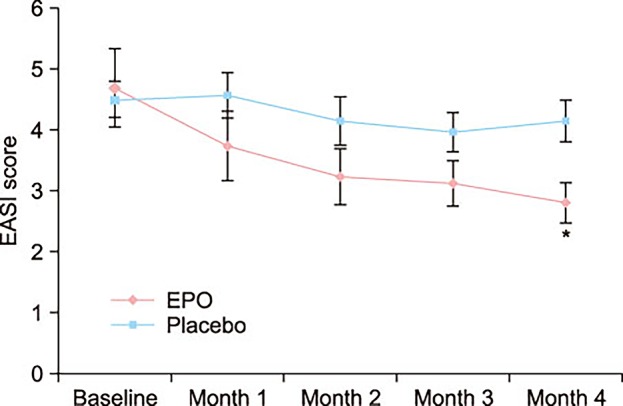
Fifty mild AD patients with an Eczema Area Severity Index (EASI) score of 10 or less were randomly divided into two groups. The first group received an oval unmarked capsule containing 450 mg of EPO (40 mg of GLA) per capsule, while placebo capsules identical in appearance and containing 450 mg of soybean oil were given to the other group. Treatment continued for a period of 4 months. EASI scores, transepidermal water loss (TEWL), and skin hydration were evaluated in all the AD patients at the baseline, and in months 1, 2, 3, and 4 of the study.
At the end of month 4, the patients of the EPO group showed a significant improvement in the EASI score, whereas the patients of the placebo group did not. There was a significant difference in the EASI score between the EPO and placebo groups. Although not statistically significant, the TEWL and skin hydration also slightly improved in the EPO patients group. Adverse effect were not found in neither the experimental group nor the control group during the study period.
The authors concluded by suggesting that EPO is a safe and effective medicine for Korean patients with mild AD.
I find this study odd for several reasons:
- One cannot possibly draw conclusions based on such a small sample.
- The authors state that a total of 69 mild AD patients were enrolled and randomized into either the control group (14 males and 17 females) or the EPO group (20 males and 18 females). Six patients in the control group and 13 patients in the EPO group dropped out due to follow up loss. No patient dropped out because the disease worsened. Should this not have necessitated an intention-to-treat analysis? And, if 19 patients were lost to follow-up, how do the authors know that their disease did not worsen?
- The graph shows impressively the lack of a placebo-response. I don’t understand why there was none.
- The authors state that there were no adverse effects at all. I find this implausible; we know that even taking placebos will prompt patients to report adverse effects.
So, what to make out of this?
I am not at all sure, but one thing is certain: this study does not alter my verdict on EPO; as far as I am concerned, the effectiveness of EPO for AD is unproven.