risk/benefit
The current secondary analysis based on the WHO database (VigiBase) of individual case safety reports (ICSRs) focuses on the suspected cutaneous adverse drug reactions (ADRs) linked to traditional medicines (TMs).
All the ICSRs reported between 1st January 2016 and 30th June 2021 from the UN Asia region in VigiBase where at least one TM was suspected to cause cutaneous ADRs were included in the study. Data regarding demographic details, suspected drug, adverse reaction as per MedDRA term, the seriousness of the reaction, de-challenge, re-challenge, and clinical outcome for suspected cutaneous ADRs associated with TM were obtained from VigiBase and analyzed for frequency of reported events and suspected medicines.
A total of 3,523 ICSRs with 5,761 ADRs related to “skin and subcutaneous tissue disorders” were included in the analysis. Amongst these, 6.8% of ICSRs were reported as serious.
The most common ADRs were:
- pruritus (29.6%),
- rash (20.3%),
- urticaria (18.9%),
- hyperhidrosis (3.3%).
Artemisia argyi H.Lév. and Vaniot. (14.9%), Ginkgo biloba L. (5.1%), Vitis vinifera L. (4%), Vitex agnus-castus L. (3.8%), Silybum marianum (L.), Gaertn (3.5%), and Viscus album L. (2.7%) were some commonly suspected TMs for cutaneous ADRs. There were 46 cases of Stevens-Johnson syndrome and toxic epidermal necrolysis reported with TMs during the study period. Death was reported in 5 ICSRs.
The authors concluded that TMs are linked with various cutaneous ADRS ranging from pruritus to toxic epidermal necrolysis which may have serious consequences. TMs listed as suspected offending agents in this analysis, should be kept in mind while dealing with suspected cutaneous ADRs. Clinicians should be more vigilant in detecting and reporting events associated with TMs.
Herbal remedies have a reputation for being time-tested, gentle, harmless, and benign. Reports such as this one might make us doubt this cliche. More importantly, they should force us to ask whether the remedy we are tempted to try truly does generate more good than harm. In most instances, I fear, the answer is not positive.
Although the use of so-called alternative medicine (SCAM) is said to be rising among older adults, many do
not discuss these healthcare practices with their primary care practitioners (PCPs). This recent US survey sought to determine the prevalence of SCAM use and to identify factors associated with SCAM disclosure among patients ages 65 and older.
Participants completed an anonymous survey, which evaluated their SCAM use over the past year and disclosure of SCAM to a PCP. Additional questions queried demographics, patient health, and relationships with one’s PCP. Analyses included descriptive statistics, chi-square tests, and logistic regression.
One hundred seventy-three participants answered surveys (response rate=23%). The Main findings were as follows:
- Sixty percent reported the use of at least one form of SCAM in the past year.
- Among those using SCAM, 64% disclosed use to their PCP.
- Patients disclosed supplements/herbal products and naturopathy/homeopathy/acupuncture at a higher rate than bodywork techniques and mind-body practices (71.9% and 66.7% vs. 48% and 50%).
- The only factor significantly associated with disclosure was trust in one’s PCP (odds ratio=2.97; confidence interval=1.01–8.73).
- The most commonly used types of SCAM were herbal products/dietary supplements (37.0%), mind-body therapies (28.9%), bodywork techniques (26.6%), and naturopathy/acupuncture/homeopathy (8.7%).
The authors concluded that clinicians may improve SCAM disclosure rates in older adults by inquiring about all types of SCAM and continuing to invest in their patient relationships, specifically by building trust.
The one-year prevalence of SCAM use – 60% – is extraordinary and considerably higher than in other surveys. How can this be explained?
I think that two factors might have played a role: firstly the survey was tiny, and secondly, its response rate was dismal. People who have no interest in SCAM would probably have not responded. Thus the prevalence figure is way too high and the survey is not representative of any population.
Having said that, I believe that some of the conclusions are still correct. As I have pointed out so often already:
- doctors need to ask their patients about SCAM usage;
- once they have identified a SCAM user, they need to advise him/her responsibly;
- to do that, they need to know about SCAM;
- as most doctors have little knowledge about the subject, they need to learn;
- failing to do that is not ethical behavior.
At first glance, the article entitled ‘Homeopathy: A State of the Science Review With Recommendations for Practical Therapies in Midwifery Practice‘ looks interesting and fairly solid; it was published in a mainstream, peer-reviewed midwifery journal; it is lengthy and thus seems thorough; it cites 125 references; and its two American authors have respectable affiliations (Art of Nursing Care Inc., Playa del Ray, California. Sonoran University of Health Sciences, Tempe, Arizona.). Yet, it does not take long to discover that ‘solid’ is not the term to describe it accurately. In fact, the paper is one of the worst examples of pseudo-science that I have ever come across. Let me just show you its conclusions:
This state of the science review has explored the history of homeopathy, its evidence base, manufacturing, regulation, and licensure. We have examined some of the controversies between homeopathy and conventional medicine in an effort to provide an overview and understanding of homeopathic science. Suggestions for practical therapies for use in midwifery practice have been given.
Despite misperceptions, homeopathy has become a well-established global practice with a growing body of research to support its benefits. Homeopathic medicines provide a comprehensive treatment approach to the myriad of conditions encountered in the midwifery practice model of care. With homeopathy’s generally accepted safety profile, low risk of side effects, few drug interactions, and low risk of overdose, midwives educated in homeopathic science can be confident that homeopathy provides a satisfactory complement for patients seeking alternative practices.
Increased opportunities for clinical research of homeopathic medicines by large funding organizations is recommended to advance patient care, understanding, and acceptance of the whole person and inform future health policy. Researchers around the world have begun to investigate the unanswered questions verifying the safety and efficacy of homeopathic treatment and the future of homeopathic research is promising. As homeopathic science continues to evolve, many health care professionals, including midwives, now seem open to adding homeopathy to complement their system of care for the whole person.
_______________________
In the article, we find two short paragraphs dealing with the effectiveness of homeopathy:
Essential to these debates are questions surrounding theories of homeopathy, such as the Law of Minimum Dose, like cures like, nonstandardized dosing, and symptom evaluation in a manner different from that of conventional medicine. It has been argued that the homeopathic paradigm is different from conventional scientific concepts associated with evidence-based medicine such as independent replication, confirmation of findings, measurement, and interpretation of results based on homeopathy’s reliance on individualized treatments and it basic tenets of the Principle of Similars and Law of Minimum Dose.69, 68 Conventional medicine practitioners find it counterintuitive that further dilution of a substance is believed to enhance its healing power when compared with a less dilute substance.65 For example, if the level of dilution is unmeasurable, how can the active ingredient be found, and is it even there?22 Recent research using nanopharmacology is beginning to uncover, identify, and characterize these ingredients in ultradiluted remedies and may help to answer these questions.39, 70 Debates arise concerning why individuals with similar symptoms often receive different treatments.22 Others ask whether homeopathic remedies perhaps inadvertently lead consumers to forgo conventional treatments that have been proven to work.5, 21, 22, 65
Interestingly, studies examining placebo therapies have appeared in scientific literature with increasing frequency, and some have compared the effectiveness of placebos with homeopathic remedies.68, 71–73 Multiple studies that have examined homeopathic treatments have found them equivalent to or no more effective than placebo,65, 68 whereas other studies found either measurable success or that patients perceived their outcomes as improved following homeopathic treatment.26, 75, 74 Mathie et al conducted a systematic review and meta-analysis focused on randomized controlled trials of nonindividualized homeopathic treatments. Authors reported that the quality of evidence was too low to determine whether homeopathic treatment results were distinguishable from those of placebo.72 These issues cited above represent some of the inconsistencies surrounding the theoretical basis and effectiveness of homeopathic therapies.
WHY WOULD ANY RESPECTABLE AUTHOR WRITE SUCH MISLEADING NONSENSE?
WHY WOULD ANY RESPECTABLE JOURNAL PUBLISH IT?
The answers to these questions might be found at the end of the paper:
Support for this supplement has been provided by Boiron USA. Boiron representatives provided no input into the article content.
Sharon Bond, CNM, PhD, who was an Associate Editor of the Journal of Midwifery & Women’s Health during the initial drafting of the manuscript, received compensation from Boiron USA for the assistance she provided the authors with editing and proofreading of the manuscript. Dr. Bond was not involved in the editorial review of or decision to publish this article.
The findings and conclusions in this supplement are those of the authors and do not necessarily reflect the official position of the host organizations, the American College of Nurse-Midwives, John Wiley & Sons, Inc., or the opinions of the journal editors.
I would argue that publishing such an article is unethical and amounts to scientific misconduct!
Enthusiasts of so-called alternative medicine (SCAM) sometimes remind me of the French philosopher, Blaire Pascal, and his famous wager. Blaise Pascal (1623-1662) argued that, because it is impossible to either prove or disprove the existence of God, it would probably be best to wager in favor of his existence. In case one got it wrong, little would be lost; in case one was correct, everything was gained.
Likewise, enthusiasts of SCAM often argue that, because of the lack of evidence for many SCAMs, one cannot be sure whether they work or not. Thus it would probably be best to wager in favor of SCAM and make use of it. In case one got it wrong, little would be lost; in case one was correct, everything was gained.
This line of thinking is common and, at first glance, it seems to be “a safe bet”. However, once we analyze it critically, it quickly falls apart. To explain, it might be best to choose a concrete example. Let’s assume, therefore, that we are talking about a cancer patient who wants to leave no stone unturned to cure her cancer.
So, she goes on the Internet and does her ‘research’. As soon as she has found a SCAM that might suit her, another one crops up, and then another, and then dozens. Which SCAM should she use? There are hundreds of SCAM cancer “cures” being promoted to the unsuspecting and vulnerable. Since one is as unproven as the next, our patient has a hard time deciding which SCAM to try. Applying all simultaneously or consecutively would be “betting on the safe side,” but is not a realistic option. If nothing else, it would be an unaffordable full-time job.
A further flaw in Pascal’s approach to SCAM relates to the fact that we are unable to prove the existence of God, but scientists are entirely capable of finding out about SCAM and its effects on cancer patients. After all, that’s what clinical trials are designed for. If for a particular SCAM, no studies are available (which is often the case), it probably means that it is not worth the effort of testing the claims that are being made for it. SCAM cancer cures are ‘alternatives’ for one main reason: they are implausible, so much so that the chances of them doing more good than harm usually approach zero.
And there is yet another caveat: while accepting the existence of God might be not associated with major harm (I know, some people would dispute this), many SCAMs are by no means free of risks. Therefore it is simply not true to assume that “little is lost” in case they do not work.
Direct harm can occur through the interactions of some form of SCAM with prescription drugs, for instance. But the potential for indirect harm is much more important. Here the risks range from raising false hopes or financial exploitation to undermining rationality in a much more general sense. By far the biggest indirect risk is that SCAM is used as a replacement for effective treatments. Most patients do not approach SCAM to give up conventional medicine entirely. But SCAM practitioners can be most persuasive, and some over-enthusiastic SCAM therapists do try to convince their patients to abandon life-saving treatments.
Pascal’s wager was disputed when it was first published. As a result of the ensuing discussions, significant advances were made, for instance, in the area of probability theory. Applying Pascal’s wager to SCAM, as many enthusiasts do, is however a very different matter. I am afraid, the benefits of doing so might not outweigh the risks.
This survey evaluated the attitude of healthcare professionals toward the use of so-called alternative medicine (SCAM) to improve current care. A questionnaire on the current practice and opinions about SCAM use was sent to healthcare professionals in Amsterdam UMC, who work for the department of hematology or oncology. Oncologists, hematologists, residents, (specialized) nurses, dieticians, (hospital)pharmacists, and pharmacy technicians were asked to participate.
Among eligible healthcare professionals, 77 responded to the questionnaire (34%). Overall, 87% of healthcare
professionals indicated it is important to be aware of their patient’s SCAM use, and all find the potential of drug–herb interactions important. However, more than half of the healthcare professionals inquire about the patient’s SCAM use infrequently. In addition, only 15% of the healthcare professionals stated they had sufficient knowledge of SCAM to advise patients on their use of SCAM.
The authors concluded that healthcare professionals are aware of the potential risks of SCAM use in combination with anti-cancer treatment. However, SCAM use is not yet discussed with every patient. This may be due to healthcare professionals’ lack of knowledge about SCAM.
This survey would in itself be fairly irrelevant; it employed only a tiny convenience sample and its findings cannot be generalized. Yet, it produced results that have been shown dozens of times before, and it might therefore be a good idea to remind ourselves of their relevance and implications.
- Patients use SCAM whether we want it or not.
- Contrary to what is often said, SCAM is not harmless.
- Therefore conventional healthcare professionals need to know about their patients’ SCAM use.
- To find out, healthcare professionals need to ask specific questions about SCAM.
- Next, they must advise their patients responsibly (this is an ethical obligation, not a choice).
- In order to do that they need to learn the essentials about SCAM.
- Failing to do this means failing their patients.
The General Chiropractic Council (GCC) “regulates chiropractors in the UK to ensure the safety of patients undergoing chiropractic treatment”. One might have assumed that they thus fulfill the important role of controlling the profession. Yet, one would have assumed wrongly. Instead of controlling, the GCC usually prefers promoting the profession. Their recent Chiropractic Patient Satisfaction and Experience is a good example. Let me show you several important sections of this document:
The outcomes reported here highlight two key findings:
• Overwhelmingly, chiropractic patients report high levels of satisfaction and positive experiences with their care. This was true both in the literature that examined international patient cohorts as well as the specific data collected from UK based chiropractic patients.
• A strong therapeutic relationship and good communication between patient and chiropractor underpins high satisfaction scores and a positive experience. This was confirmed both in the international literature and through both quantitative and qualitative analysis of specific data collected from UK based chiropractic patients.
Conclusion
This report shows that both existing literature and de novo data collection from patients receiving chiropractic care in the UK highlight excellent perceived experience and high satisfaction with such care.
Factors such as therapeutic alliance and communication are strongly associated with these positive perceptions by patients although other factors such as treatment beliefs were also significantly associated with satisfaction scores.
Recommendations
• To offer the highest quality of care, both in terms of clinical outcomes and patient experience, chiropractors should be explicitly skilled at curating excellent therapeutic alliances and communication with patients.
• Such skills and competences within chiropractic care delivery should receive higher visibility within the chiropractic profession generally and more specifically through advocacy within leading institutions and core emphasis within chiropractic curricula.
__________________________
By changing a few words, I have adapted the above excerpts to become a Customer Satisfaction and Experience Report of a fictitious hamburger joint published by the Hamburger General Council (HGC) of Great Britain which regulates hamburger joints in the UK to ensure the safety of consumers undergoing hamburger nutrition:
The outcomes reported here highlight two key findings:
• Overwhelmingly, customers report high levels of satisfaction and positive experiences with their restaurant. This was true both in the literature that examined international consumer cohorts as well as the specific data collected from UK based customers.
• A strong professional relationship and good communication between customer and service personell underpins high satisfaction scores and a positive experience. This was confirmed both in the international literature and through both quantitative and qualitative analysis of specific data collected from UK based hamburger consumers.
Conclusion
This report shows that both existing literature and de novo data collection from consumers eating hamburgers in the UK highlight excellent perceived experience and high satisfaction with such service.
Factors such as personal alliance and communication are strongly associated with these positive perceptions by consumers although other factors such as appetite were also significantly associated with satisfaction scores.
Recommendations
• To offer the highest quality of service, both in terms of profit and patient experience, hamburger vendors should be explicitly skilled at curating excellent professional alliances and communication with customers.
• Such skills and competences within hamburger delivery should receive higher visibility within the gastronomic trade generally and more specifically through advocacy within leading institutions and core emphasis within servers’ curricula.
___________________________
If you get the impression that I am taking the Mickey of the GCC, you are not mistaken. Yet, this post also has slightly more serious purposes. I wanted to 1) show how, in the chiropractic profession, pure BS is often disguised as research, and 2) question whether the GCC is fit for purpose.
On a more constructive note: there are many open questions that urgently need addressing in the realm of chiropractic (e.g. do chiropractors more good than harm?). I, therefore, suggest that the GCC stops publishing idiotic promotional documents disguised as research and gets on with its responsibilities.
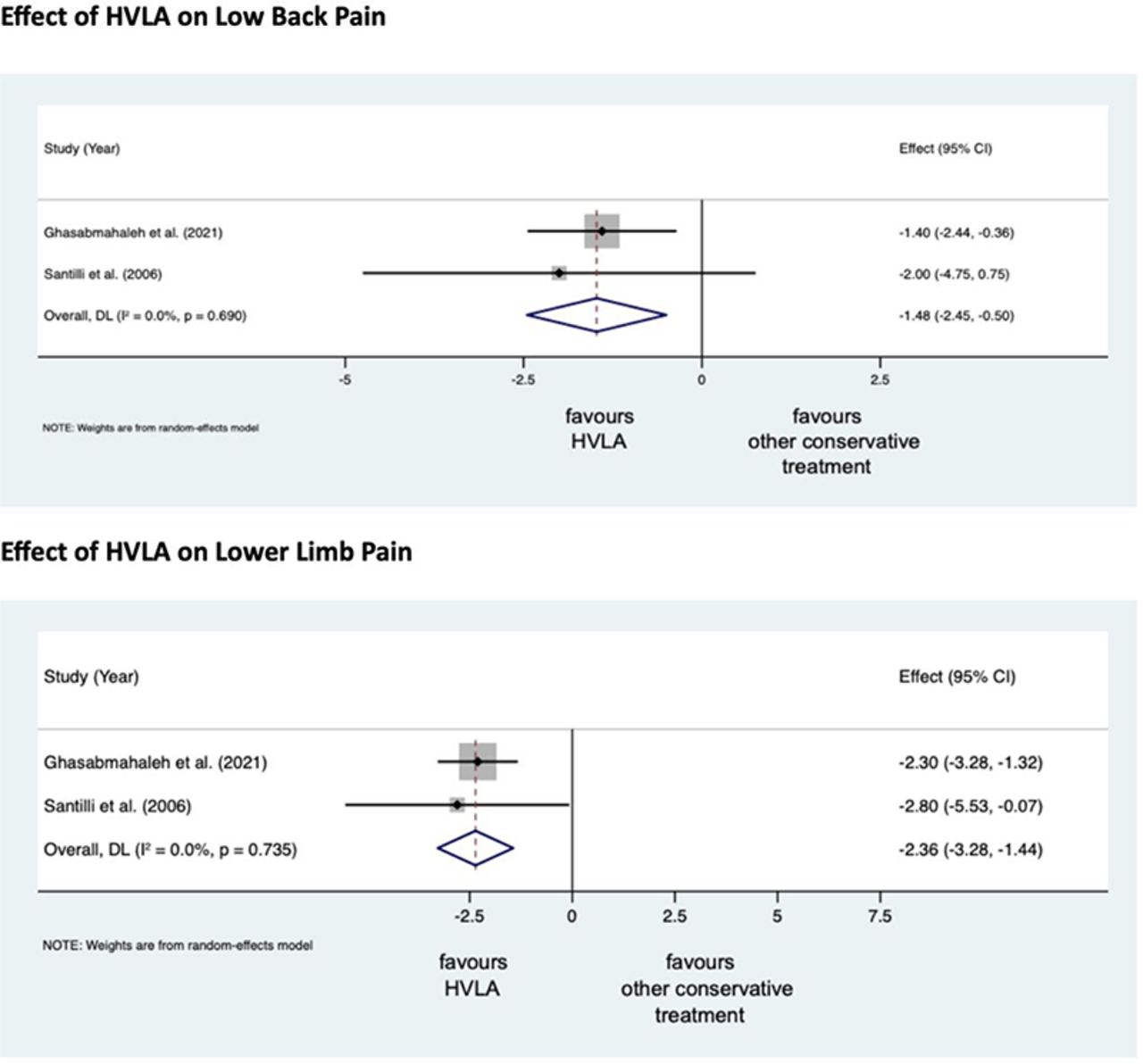
Lumbosacral Radicular Syndrome (LSRS) is a condition characterized by pain radiating in one or more dermatomes (Radicular Pain) and/or the presence of neurological impairments (Radiculopathy). So far, different reviews have investigated the effect of HVLA (high-velocity low-amplitude) spinal manipulations in LSRS. However, these studies included ‘mixed’ population samples (LBP patients with or without LSRS) and treatments other than HVLA spinal manipulations (e.g., mobilisation, soft tissue treatment, etc.). Hence, the efficacy of HVLAT in LSRS is yet to be fully understood.
This review investigated the effect and safety of HVLATs on pain, levels of disability, and health-related quality of life in LSRS, as well as any possible adverse events.
Randomized clinical trials (RCTs) published in English in the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (PubMed), EMBASE, PEDro, and Web of Science were identified. RCTs on an adult population (18-65 years) with LSRS that compared HVLATs with other non-surgical treatments, sham spinal manipulation, or no intervention were considered. Two authors selected the studies, extracted the data, and assessed the methodological quality through the ‘Risk of Bias (RoB) Tool 2.0’ and the certainty of the evidence through the ‘GRADE tool’. A meta-analysis was performed to quantify the effect of HVLA on pain levels.
A total of 308 records were retrieved from the search strings. Only two studies met the inclusion criteria. Both studies were at high RoB. Two meta-analyses were performed for low back and leg pain levels. HVLA seemed to reduce the levels of low back (MD = -1.48; 95% CI = -2.45, -0.50) and lower limb (MD = -2.36; 95% CI = -3.28, -1.44) pain compared to other conservative treatments, at three months after treatment. However, high heterogeneity was found (I² = 0.0%, p = 0.735). Besides, their certainty of the evidence was ‘very low’. No adverse events were reported.
The authors stated that they cannot conclude whether HVLA spinal manipulations can be helpful for the treatment of LSRS or not. Future high-quality RCTs are needed to establish the actual effect of HVLA manipulation in this disease with adequate sample size and LSRS definition.
Chiropractors earn their living by applying HVLA thrusts to patients suffering from LSRS. One would therefore have assumed that the question of efficacy has been extensively researched and conclusively answered. It seems that one would have assumed wrongly!
Now that this is (yet again) in the open, I wonder whether chiropractors will, in the future, tell their patients while obtaining informed consent: “I plan to give you a treatment for which sound evidence is not available; it can also cause harm; and, of course, it will cost you – I hope you don’t mind.”
“The decline of homeopathy, the ‘medicine’ that doesn’t cure anything” is the title of a remarkable article in EL PAIS of which I take the liberty of showing you a few key passages:
In the more than 200 years that have passed since its invention, no one has been able to prove that homeopathy is actually capable of curing anything with its alleged medicines that have no active ingredients…
…EL PAÍS reached out to some of its main promoters, such as the pharmaceutical company Boiron, leader in the sector; the Spanish Association of Homeopathy Pharmacists and the Spanish Society of Homeopathic Doctors. In the absence of a response from all three, the explanations are given by experts who are more critical of the discipline.
Many people who used to consume homeopathy were not even aware that this was the case. Fernando Frías, one of the activists who worked to undermine the discipline’s remaining prestige, recalls that people did not believe them when they were told that compounds with diluted Berlin Wall were sold to overcome the feelings of oppression and anxiety. This was actually commercialized under the premise that “like cures like”: if the Berlin Wall oppressed, a piece of it diluted in water should remedy it. “Many were under the impression that it was just a natural therapy and that we were making things up to attack it,” says Frías…
… There has been a lot of debate about how to regulate an alleged drug whose only effect is, in truth, the placebo effect. In 2001, the European Parliament issued a directive that covered its use in countries with a homeopathic tradition; sources explain that this happened due to the pressure exerted by both the industries and the governments of countries where pseudoscience is deep-rooted, such as France (where Boiron is headquartered) or Germany, where its consumption is much higher than in others, such as Spain.
“Having regard to the particular characteristics of these homeopathic medicinal products, such as the very low level of active principles they contain and the difficulty of applying to them the conventional statistical methods relating to clinical trials, it is desirable to provide a special, simplified registration procedure for those homeopathic medicinal products which are placed on the market without therapeutic indications in a pharmaceutical form and dosage which do not present a risk for the patient,” states the directive.
In its more than two centuries of history, this is not the first time that homeopathy loses ground. Still, Frías warns, it cannot be ruled out that at some point something will come up that will make it fashionable again. “Look at the example of chemtrails [the condensation trails left by airplanes that some conspiracy theorists believe are a way of poisoning the population from the air]. It seemed that no one remembered them anymore, but now they’re back,” he says. Frías cites the astrophysicist and disseminator Javier Armentia, who states that beliefs are like a rubber duck: no matter how much they sink, they always resurface. “Especially if there is money behind,” he adds.
______________________
As reported previously, homeopathy and other forms of so-called alternative medicine (SCAM) have come under fire in Spain. In 2017, ‘HOMEOPATHY PLUS‘ reported that “in a reversal of the 2015 Royal Legislative Decree, the Minister of Health has withdrawn homeopathic remedies and outlawed the practice in Spain’s national health services.” In 2018, more than 400 people signed an open letter triggered by the case of a cancer patient who died after preferring homeopathy to regular treatment. “Let’s be clear: pseudoscience kills,” begins the letter. Since then, the struggle of Spanish rational thinkers to stop misleading information about SCAM in general and homeopathy, in particular, has only intensified.
Spain is thus joining other European countries in opposing misinformation about homeopathy. Contrary to what some have claimed (for instance, in the comments section of this blog), most of the opponents do not want to restrict the public’s choice. People who wish to use homeopathy should be able to do so (but should pay for it themselves). However, the choice must be based on evidence-based information.
An explanatory sequential mixed methods study with three separate phases was conducted in Danish patients with lumbar radiculopathy receiving a standardized chiropractic care package (SCCP). Lumbar radiculopathy is pain and other neurological symptoms caused by the pinching of nerve roots where they leave your spinal cord in the lumbar region. 
Phase one of the study was a quantitative analysis based on a survey in a prospective cohort of patients with lumbar radiculopathy in an SCCP from 2018 to 2020. Patients rated their satisfaction with the examination, information, treatment effect, and overall management of their problem on a 0–10 scale. In phase two, six semi-structured interviews conducted in 2021 were used to gain further explanatory insights into the findings from phase one. Data were analyzed using systematic text condensation. In phase three, the quantitative and qualitative data were merged in a narrative joint display to obtain a deeper understanding of the overall results.
Here I am only interested in the patients’ perception of the treatment effect. Of 303 eligible patients, 238 responded to the survey. Of these, 50% were very satisfied with the treatment effect.
The authors stated that patients in their study expected a rapid and persistent decrease in symptoms, which, unfortunately, does not match the prognosis of lumbar radiculopathy. Although the prognosis is considered good, the improvement happens gradually and often with fluctuating pain patterns, and it is not unusual to have milder symptoms for three months or longer.
So, only half of the patients who had chosen to consult chiropractors for their lumbar radiculopathy were very satisfied with the treatment results. In most patients, the symptoms decreased only gradually often with fluctuating pain patterns, and the authors comment that symptoms frequently last for three months or longer with a SCCP.
Impressive?
Might I point out that what is being described here looks to me very much like the natural history of lumbar radiculopathy? About 90% of patients with back pain caused by disc herniation see improvements within three months without therapy. Are the authors aware that their observational study is in accordance with the notion that the SCCP does nothing or very little to help patients suffering from lumbar radiculopathy?
On this blog, we have some people who continue to promote conspiracy theories about Covid and Covid vaccinations. It is, therefore, time, I feel, to present them with some solid evidence on the subject (even though it means departing from our usual focus on SCAM).
This Cochrane review assessed the efficacy and safety of COVID‐19 vaccines (as a full primary vaccination series or a booster dose) against SARS‐CoV‐2. An impressive team of investigators searched the Cochrane COVID‐19 Study Register and the COVID‐19 L·OVE platform (last search date 5 November 2021). They also searched the WHO International Clinical Trials Registry Platform, regulatory agency websites, and Retraction Watch. They included randomized controlled trials (RCTs) comparing COVID‐19 vaccines to placebo, no vaccine, other active vaccines, or other vaccine schedules.
A total of 41 RCTs could be included and analyzed assessing 12 different vaccines, including homologous and heterologous vaccine schedules and the effect of booster doses. Thirty‐two RCTs were multicentre and five were multinational. The sample sizes of RCTs were 60 to 44,325 participants. Participants were aged: 18 years or older in 36 RCTs; 12 years or older in one RCT; 12 to 17 years in two RCTs; and three to 17 years in two RCTs. Twenty‐nine RCTs provided results for individuals aged over 60 years, and three RCTs included immunocompromised patients. No trials included pregnant women. Sixteen RCTs had two‐month follow-ups or less, 20 RCTs had two to six months, and five RCTs had greater than six to 12 months or less. Eighteen reports were based on preplanned interim analyses. The overall risk of bias was low for all outcomes in eight RCTs, while 33 had concerns for at least one outcome. 343 registered RCTs with results not yet available were identified.The evidence for mortality was generally sparse and of low or very low certainty for all WHO‐approved vaccines, except AD26.COV2.S (Janssen), which probably reduces the risk of all‐cause mortality (risk ratio (RR) 0.25, 95% CI 0.09 to 0.67; 1 RCT, 43,783 participants; high‐certainty evidence).High‐certainty evidence was found that BNT162b2 (BioNtech/Fosun Pharma/Pfizer), mRNA‐1273 (ModernaTx), ChAdOx1 (Oxford/AstraZeneca), Ad26.COV2.S, BBIBP‐CorV (Sinopharm‐Beijing), and BBV152 (Bharat Biotect) reduce the incidence of symptomatic COVID‐19 compared to placebo (vaccine efficacy (VE): BNT162b2: 97.84%, 95% CI 44.25% to 99.92%; 2 RCTs, 44,077 participants; mRNA‐1273: 93.20%, 95% CI 91.06% to 94.83%; 2 RCTs, 31,632 participants; ChAdOx1: 70.23%, 95% CI 62.10% to 76.62%; 2 RCTs, 43,390 participants; Ad26.COV2.S: 66.90%, 95% CI 59.10% to 73.40%; 1 RCT, 39,058 participants; BBIBP‐CorV: 78.10%, 95% CI 64.80% to 86.30%; 1 RCT, 25,463 participants; BBV152: 77.80%, 95% CI 65.20% to 86.40%; 1 RCT, 16,973 participants).Moderate‐certainty evidence was found that NVX‐CoV2373 (Novavax) probably reduces the incidence of symptomatic COVID‐19 compared to placebo (VE 82.91%, 95% CI 50.49% to 94.10%; 3 RCTs, 42,175 participants).There is low‐certainty evidence for CoronaVac (Sinovac) for this outcome (VE 69.81%, 95% CI 12.27% to 89.61%; 2 RCTs, 19,852 participants).High‐certainty evidence was found that BNT162b2, mRNA‐1273, Ad26.COV2.S, and BBV152 result in a large reduction in the incidence of severe or critical disease due to COVID‐19 compared to placebo (VE: BNT162b2: 95.70%, 95% CI 73.90% to 99.90%; 1 RCT, 46,077 participants; mRNA‐1273: 98.20%, 95% CI 92.80% to 99.60%; 1 RCT, 28,451 participants; AD26.COV2.S: 76.30%, 95% CI 57.90% to 87.50%; 1 RCT, 39,058 participants; BBV152: 93.40%, 95% CI 57.10% to 99.80%; 1 RCT, 16,976 participants).
Moderate‐certainty evidence was found that NVX‐CoV2373 probably reduces the incidence of severe or critical COVID‐19 (VE 100.00%, 95% CI 86.99% to 100.00%; 1 RCT, 25,452 participants).
Two trials reported high efficacy of CoronaVac for severe or critical disease with wide CIs, but these results could not be pooled.
mRNA‐1273, ChAdOx1 (Oxford‐AstraZeneca)/SII‐ChAdOx1 (Serum Institute of India), Ad26.COV2.S, and BBV152 probably result in little or no difference in serious adverse events (SAEs) compared to placebo (RR: mRNA‐1273: 0.92, 95% CI 0.78 to 1.08; 2 RCTs, 34,072 participants; ChAdOx1/SII‐ChAdOx1: 0.88, 95% CI 0.72 to 1.07; 7 RCTs, 58,182 participants; Ad26.COV2.S: 0.92, 95% CI 0.69 to 1.22; 1 RCT, 43,783 participants); BBV152: 0.65, 95% CI 0.43 to 0.97; 1 RCT, 25,928 participants). In each of these, the likely absolute difference in effects was fewer than 5/1000 participants.
Evidence for SAEs is uncertain for BNT162b2, CoronaVac, BBIBP‐CorV, and NVX‐CoV2373 compared to placebo (RR: BNT162b2: 1.30, 95% CI 0.55 to 3.07; 2 RCTs, 46,107 participants; CoronaVac: 0.97, 95% CI 0.62 to 1.51; 4 RCTs, 23,139 participants; BBIBP‐CorV: 0.76, 95% CI 0.54 to 1.06; 1 RCT, 26,924 participants; NVX‐CoV2373: 0.92, 95% CI 0.74 to 1.14; 4 RCTs, 38,802 participants).
The authors’ conclusions were as follows: Compared to placebo, most vaccines reduce, or likely reduce, the proportion of participants with confirmed symptomatic COVID‐19, and for some, there is high‐certainty evidence that they reduce severe or critical disease. There is probably little or no difference between most vaccines and placebo for serious adverse events. Over 300 registered RCTs are evaluating the efficacy of COVID‐19 vaccines, and this review is updated regularly on the COVID‐NMA platform (covid-nma.com).
_____________________
As some conspiratorial loons will undoubtedly claim that this review is deeply biased; it might be relevant to add the conflicts of interest of its authors:
- Carolina Graña: none known.
- Lina Ghosn: none known.
- Theodoros Evrenoglou: none known.
- Alexander Jarde: none known.
- Silvia Minozzi: no relevant interests; Joint Co‐ordinating Editor and Method editor of the Drugs and Alcohol Group.
- Hanna Bergman: Cochrane Response – consultant; WHO – grant/contract (Cochrane Response was commissioned by the WHO to perform review tasks that contribute to this publication).
- Brian Buckley: none known.
- Katrin Probyn: Cochrane Response – consultant; WHO – consultant (Cochrane Response was commissioned to perform review tasks that contribute to this publication).
- Gemma Villanueva: Cochrane Response – employment (Cochrane Response has been commissioned by WHO to perform parts of this systematic review).
- Nicholas Henschke: Cochrane Response – consultant; WHO – consultant (Cochrane Response was commissioned by the WHO to perform review tasks that contributed to this publication).
- Hillary Bonnet: none known.
- Rouba Assi: none known.
- Sonia Menon: P95 – consultant.
- Melanie Marti: no relevant interests; Medical Officer at WHO.
- Declan Devane: Health Research Board (HRB) – grant/contract; registered nurse and registered midwife but no longer in clinical practice; Editor, Cochrane Pregnancy and Childbirth Group.
- Patrick Mallon: AstraZeneca – Advisory Board; spoken of vaccine effectiveness to media (print, online, and live); works as a consultant in a hospital that provides vaccinations; employed by St Vincent’s University Hospital.
- Jean‐Daniel Lelievre: no relevant interests; published numerous interviews in the national press on the subject of COVID vaccination; Head of the Department of Infectious Diseases and Clinical Immunology CHU Henri Mondor APHP, Créteil; WHO (IVRI‐AC): expert Vaccelarate (European project on COVID19 Vaccine): head of WP; involved with COVICOMPARE P et M Studies (APHP, INSERM) (public fundings).
- Lisa Askie: no relevant interests; Co‐convenor, Cochrane Prospective Meta‐analysis Methods Group.
- Tamara Kredo: no relevant interests; Medical Officer in an Infectious Diseases Clinic at Tygerberg Hospital, Stellenbosch University.
- Gabriel Ferrand: none known.
- Mauricia Davidson: none known.
- Carolina Riveros: no relevant interests; works as an epidemiologist.
- David Tovey: no relevant interests; Emeritus Editor in Chief, Feedback Editors for 2 Cochrane review groups.
- Joerg J Meerpohl: no relevant interests; member of the German Standing Vaccination Committee (STIKO).
- Giacomo Grasselli: Pfizer – speaking engagement.
- Gabriel Rada: none known.
- Asbjørn Hróbjartsson: no relevant interests; Cochrane Methodology Review Group Editor.
- Philippe Ravaud: no relevant interests; involved with Mariette CORIMUNO‐19 Collaborative 2021, the Ministry of Health, Programme Hospitalier de Recherche Clinique, Foundation for Medical Research, and AP‐HP Foundation.
- Anna Chaimani: none known.
- Isabelle Boutron: no relevant interests; member of Cochrane Editorial Board.
___________________________
And as some might say this analysis is not new, here are two further papers just out:
Objectives To determine the association between covid-19 vaccination types and doses with adverse outcomes of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection during the periods of delta (B.1.617.2) and omicron (B.1.1.529) variant predominance.
Design Retrospective cohort.
Setting US Veterans Affairs healthcare system.
Participants Adults (≥18 years) who are affiliated to Veterans Affairs with a first documented SARS-CoV-2 infection during the periods of delta (1 July-30 November 2021) or omicron (1 January-30 June 2022) variant predominance. The combined cohorts had a mean age of 59.4 (standard deviation 16.3) and 87% were male.
Interventions Covid-19 vaccination with mRNA vaccines (BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna)) and adenovirus vector vaccine (Ad26.COV2.S (Janssen/Johnson & Johnson)).
Main outcome measures Stay in hospital, intensive care unit admission, use of ventilation, and mortality measured 30 days after a positive test result for SARS-CoV-2.
Results In the delta period, 95 336 patients had infections with 47.6% having at least one vaccine dose, compared with 184 653 patients in the omicron period, with 72.6% vaccinated. After adjustment for patient demographic and clinical characteristics, in the delta period, two doses of the mRNA vaccines were associated with lower odds of hospital admission (adjusted odds ratio 0.41 (95% confidence interval 0.39 to 0.43)), intensive care unit admission (0.33 (0.31 to 0.36)), ventilation (0.27 (0.24 to 0.30)), and death (0.21 (0.19 to 0.23)), compared with no vaccination. In the omicron period, receipt of two mRNA doses were associated with lower odds of hospital admission (0.60 (0.57 to 0.63)), intensive care unit admission (0.57 (0.53 to 0.62)), ventilation (0.59 (0.51 to 0.67)), and death (0.43 (0.39 to 0.48)). Additionally, a third mRNA dose was associated with lower odds of all outcomes compared with two doses: hospital admission (0.65 (0.63 to 0.69)), intensive care unit admission (0.65 (0.59 to 0.70)), ventilation (0.70 (0.61 to 0.80)), and death (0.51 (0.46 to 0.57)). The Ad26.COV2.S vaccination was associated with better outcomes relative to no vaccination, but higher odds of hospital stay and intensive care unit admission than with two mRNA doses. BNT162b2 was generally associated with worse outcomes than mRNA-1273 (adjusted odds ratios between 0.97 and 1.42).
Conclusions In veterans with recent healthcare use and high occurrence of multimorbidity, vaccination was robustly associated with lower odds of 30 day morbidity and mortality compared with no vaccination among patients infected with covid-19. The vaccination type and number of doses had a significant association with outcomes.
SECOND EXAMPLE Long COVID, or complications arising from COVID-19 weeks after infection, has become a central concern for public health experts. The United States National Institutes of Health founded the RECOVER initiative to better understand long COVID. We used electronic health records available through the National COVID Cohort Collaborative to characterize the association between SARS-CoV-2 vaccination and long COVID diagnosis. Among patients with a COVID-19 infection between August 1, 2021 and January 31, 2022, we defined two cohorts using distinct definitions of long COVID—a clinical diagnosis (n = 47,404) or a previously described computational phenotype (n = 198,514)—to compare unvaccinated individuals to those with a complete vaccine series prior to infection. Evidence of long COVID was monitored through June or July of 2022, depending on patients’ data availability. We found that vaccination was consistently associated with lower odds and rates of long COVID clinical diagnosis and high-confidence computationally derived diagnosis after adjusting for sex, demographics, and medical history.
_______________________________________
There are, of course, many more articles on the subject for anyone keen to see the evidence. Sadly, I have little hope that the COVID loons will be convinced by any of them. Yet, I thought I should give it nevertheless a try.