prevention
Ischemic heart disease (IHD) related to cardiovascular or cerebrovascular disease is the leading cause of mortality and an important issue of public health worldwide. The cost of long-term healthcare for IHD patients may result in a huge financial burden. This study analyzed the medical expenditure incurred for and survival of IHD patients treated with Chinese herbal medicine (CHM) and Western medicine.
Subjects were randomly selected from the National Health Insurance Research Database in Taiwan. The Cox proportional hazards regression model, Kaplan–Meier estimator, logrank test, chi-square test, and analysis of variance were applied. Landmark analysis was used to assess the cumulative incidence of death in IHD patients.
A total of 11,527 users were identified as CHM combined with Western medicine and 11,527 non-CHM users. CHM users incurred a higher medical expenditure for outpatient care within 1 (24,529 NTD versus 18,464 NTD, value <0.0001) and 5 years (95,345 NTD versus 60,367 NTD, value <0.0001). However, CHM users had shorter hospitalizations and lower inpatient medical expenditure (7 days/43,394 NTD in 1 year; 11 days/83,141 NTD in 5 years) than non-CHM users (11 days/72,939 NTD in 1 year; 14 days/107,436 NTD in 5 years).
The CHM group’s adjusted hazard ratio for mortality was 0.41 lower than that of the non-CHM group by Cox proportional hazard models with time-dependent exposure covariates. Danshen, Huang qi, Niu xi, Da huang, and Fu zi were the most commonly prescribed Chinese single herbs; Zhi-Gan-Cao-Tang, Xue-Fu-Zhu-Yu-Tang, Tian-Wang-Bu-Xin-Dan, Sheng-Mai-San, and Yang-Xin-Tang were the five most frequently prescribed herbal formulas in Taiwan.
The authors concluded that combining Chinese and Western medicine can reduce hospital expenditure and improve survival for IHD patients.
Why, you will ask, do I think that this study deserves to be in the ‘worst paper cometition’?
It is not so bad!
It is an epidemiological case-control study with a large sample size that generates interesting findings.
Agreed!
But, as a case-control study, it cannot establish a causal link between CHM and the outcomes. You might argue that the conclusions avoid doing this – “can … improve survival” is not the same as “does improve survival”. This may be true, yet the title of the article leaves little doubt about the interpretation of the authors:
Chinese Herbal Medicine as an Adjunctive Therapy Improves the Survival Rate of Patients with Ischemic Heart Disease: A Nationwide Population-Based Cohort Study
I find it difficult not to view this as a deliberate attempt of the authors, editors, and reviewers to mislead the public.
Looking at the details of the study, it is easy to see that the two groups were different in a whole range of parameters that were measured. More importantly, they most likely differ in a range of variables that were not measured and had significant influence on IHD survival. It stands to reason, for instance, that patients who elected to use CHM in addition to their standard care were more health conscious. They would thus have followed a healthier diet and lifestyle. It would be foolish to claim that such factors do not influence IHD survival.
The fact that the authors fail even to mention this possibility, interpret an association as a causal link, and thus try to mislead us all makes this paper, in my view, a strong contender for my
WORST PAPER OF 2022 COMPETITION
This cohort study was designed as undertaken to evaluate the protective effect of Arsenicum album 30C against COVID-19.
Participants were enrolled in a homeopathy intervention (HI) cohort (who received Arsenicum album) or in a non-intervention (NI) cohort (who received no systematic intervention) from COVID-19 containment areas of Delhi. Individuals of age 5 years or above were given four medicated pills of Arsenicum album 30C, while those from 1 to 5 years old were given two medicated pills in each dose.
The analysis included 10,180 individuals residing in 11 COVID-19 containment areas in Delhi, out of which 6,590 individuals were in the HI cohort and 3,590 individuals were in the NI cohort. The overall protective effect of Arsenicum album 30C was 83% (95% confidence interval [CI], 76.77 to 88.17): 45 cases per 6,590 (8.34 per 10,000 person-weeks) in the Arsenicum album 30C group versus 143 cases per 3,590 (45.01 per 10,000 person-weeks) in the NI cohort. The protective effect of Arsenicum album 30C against laboratory-confirmed COVID-19 was 74% (95% CI, 55.08 to 85.41): 18 cases per 6,590 (3.32 per 10,000 person-weeks) in the Arsenicum album 30C group versus 38 cases per 3,590 (11.85 per 10,000 person-weeks) in the NI cohort.
The authors concluded that the use of Arsenicum album 30C was associated with some protection against probable and laboratory-confirmed COVID-19 in a containment-zone setting. Randomized controlled trials are needed to confirm or refute these results.
It is remarkable, I feel, that the authors conclude Arsenicum album 30C was associated with some protection. All too often enthusiasts of homeopathy claim a causal link where there is none – but not this time, and I wonder why.
Unfortunately, I was unable to read the full text of the paper (it’s behind a paywall and I would be grateful for anyone to make it available). Thus, I cannot comment on one of the most crucial questions related to the study: how were the patients divided into the two groups?
It is clear that it was not by randomization. Yet only randomization would have created two fully comparable groups. The most likely explanation for the findings of this trial is therefore that the two groups differed in terms of one or more prognostic factors. This would explain why a group of patients receiving a placebo (Arsenicum album C30 is a dilution of Arsenic at a ratio of 1: 1000000000000000000000000000000000000000000000000000000000000 and therefore is a pure placebo [unless, of course, one believes in homeopathic magic) experience different outcomes from those receiving nothing.
As I said, the answer can only be found by studying the precise selection criteria used in this study. Until this is cleared up, I can only say three things for sure:
- A causal link between the treatment and the result is highly unlikely.
- It is regrettable that researchers do not use randomization for potentially important trials.
- It seems unethical to encourage placebo use for the prevention of a serious illness.
_______________________
UPDATE
I just received the full text from one of the authors. This is what they say about the allocation of the participants:
“Participants were enrolled in two cohorts: the homeopathy intervention (HI) cohort and the non-intervention (NI) cohort. Recruitment to the cohorts was at cluster level (containment area): the clusters were allotted to the HI cohort or the NI cohort as per convenience.”
I am afraid, this tells me very little, and my concerns noted above still apply.
A few other points are of relevance:
- The study was conducted between April and August 2020. This begs the question of why it took 2 years to publish the findings.
- The outcomes were verified via telephone. This means that social desirability might have influenced the results.
- The paper also confirms that there were many important differences between the groups that might have prognostic significance.
- The conclusion at the end of the paper does imply causality in stronger terms than the abstract: “The use of Arsenicum album 30C may help protect against COVID-19 infection. Randomized controlled trials are needed
to confirm or refute our findings.
Due to polypharmacy combined with the rising popularity of so-called alternative medicines (SCAM), oncology patients are at particular risk of drug-drug interactions (DDI) or herb-drug interactions (HDI). Caution is therefore indicated.
The aims of this study were to assess DDI and HDI in outpatients taking oral anticancer drugs.
All prescribed and non-prescribed medications, including SCAM, were prospectively collected by hospital pharmacists during a structured interview with the patient. DDI and HDI were analyzed using four interaction software programs: Thériaque®, Drugs.com®, Hédrine, and Memorial Sloan Kettering Cancer Center (MSKCC) database. All detected interactions were characterized by severity, risk, and action mechanism. The need for pharmaceutical intervention to modify drug use was determined on a case-by-case basis.
294 patients were included, with a mean age of 67 years. The median number of chronic drugs per patient was 8 [1-29] and 55% of patients used at least one SCAM. At least 0ne interaction was found for 267 patients (90.8%): 263 (89.4%) with DDI, 68 (23.1%) with HDI, and 64 (21.7%) with both DDI and HDI. Only 13% of the DDI were found in Thériaque® and Drugs.com® databases, and 125 (2.5%) were reported with a similar level of risk on both databases. 104 HDI were identified with only 9.5% of the interactions found in both databases. 103 pharmaceutical interventions were performed, involving 61 patients (20.7%).
The authors concluded that potentially clinically relevant drug interactions were frequently identified in this study, showing that several databases and structured screening are required to detect more interactions and optimize medication safety.
This figure of potential HDIs is high – much higher than in most previous studies. A possible explanation could be that the study was carried out in France where the use of herbal remedies is considerable. As some HDIs can cause serious problems for patients, my advice is to think twice about using herbal remedies while taking prescription drugs. I think this advice is sound regardless of whether someone is suffering from cancer or any other condition.
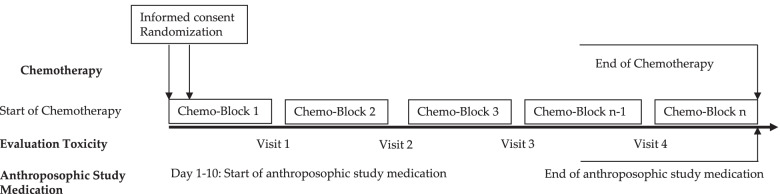
This multi-center, open-label, randomized controlled trial assessed the effects of anthroposophic treatments on toxicity related to intensive-phase chemotherapy treatment in children aged 1-18 with the primary outcome of the toxicity sum score. Secondary outcomes were chemotherapy-related toxicity, overall and event-free survival after 5 years in study patients.
The main sponsorship for the study was provided by: Helixor Heilmittel GmbH & Co. KG, Rosenfeld. Additional finacial support was provided by: WALA Heilmittel GmbH, Bad Boll/Eckwälden; Weleda AG, Schwäbisch Gmünd: Mahle Stiftung, Stuttgart; Software AG Stiftung, Darmstadt; Stiftung Helixor, Rosenfeld; and Injex Pharma AG, Berlin.
The intervention and control groups were both given standard chemotherapy according to malignancy & tumor type. The intervention arm was provided with anthroposophic supportive treatment (AST); given as anthroposophic base medication (AMP), as a base medication for all patients, and additional on-demand treatment tailored to the patient in the intervention groups. The control was given no AMP. The toxicity sum score (TSS) was assessed using NCI-CTC scales.
The AST consisted of base AMP including Helixor®, and on-demand supplementary AMP given as needed for symptoms. Administration of the AST intervention and chemotherapy protocol were tailored for each type of pediatric malignancy included in the trial. This included both the base and the on-demand AMP, which were administered based on acute symptoms during intensive chemotherapy. The intervention group started the AST between the day of randomization and day 10 of the first chemotherapy cycle.
Data of 288 patients could be analyzed. The analysis did not reveal any statistically significant differences between the AST and the control group for the primary endpoint or the toxicity measures (secondary endpoints). Furthermore, groups did not differ significantly in the five-year overall and event-free survival follow-up.
The authors concluded that their findings showed that AST was able to be safely administered in a clinical setting, although no beneficial effects of AST between group toxicity scores, overall or event-free survival were shown.
In their discussion section, the authors explain the findings more clearly: “In the long term follow up, the explorative analysis of the data available for the 5-year follow up found no indications that efficacy of chemotherapy was influenced by AST. For long-term toxicities there were also no indications of an influence of AST.”
Question: what do we call a treatment that has neither adverse nor beneficial effects?
Could it be
PLACEBO?
An epidemiological study from the US just published in the BMJ concluded that “the mortality gap in Republican voting counties compared with Democratic voting counties has grown over time, especially for white populations, and that gap began to widen after 2008.”
In a BMJ editorial, Steven Woolf comments on the study and provides further evidence on how politics influence health in the US. Here are his concluding two paragraphs:
Political influence on US mortality rates became overt during the covid-19 pandemic, when public health policies, controlled by states, were heavily influenced by party affiliation. Republican politicians, often seeking to appeal to President Trump and his supporters, challenged scientific evidence and opposed enforcement of vaccinations and safety measures such as masking. A macabre natural experiment occurred in 2021, a year marked by the convergence of vaccine availability and contagious variants that threatened unvaccinated populations: states led by governors who promoted vaccination and mandated pandemic control measures experienced much lower death rates than the “control” group, consisting of conservative states with lax policies and large unvaccinated populations. This behavior could explain why US mortality rates associated with covid-19 were so catastrophic, vastly exceeding losses in other high income countries.
Observers of health trends in the US should keep their eye on state governments, where tectonic shifts in policy are occurring. While gridlock in Washington, DC incapacitates the federal government, Republican leaders in dozens of state capitols are passing laws to undermine health and safety regulations, ban abortion, limit LGBT+ rights, and implement more conservative policies on voting, school curriculums, and climate policy. To understand the implications for population health, researchers must break with custom; although scientific literature has traditionally avoided discussing politics, the growing influence of partisan affiliation on policies affecting health makes this covariate an increasingly important subject of study.
_____________________
What has this to do with so-called alternative medicine (SCAM)?
Not a lot.
Except, of course, that Trump has been quite sympathetic to both quackery and quacks (see, for instance, here and here). Moreover, the embarrassing Dr. Oz, America’s charlatan-in-chief, is now a Republican candidate for the US senate. And the creation of the NHI office for alternative medicine, currently called NCCIH, was the idea of the Republican senator, Tom Harkin.
I think we get the drift: on the US political level, SCAM seems to be a right-wing thing.
Am I claiming that SCAM is the cause of the higher mortality in Republican counties?
No.
Do I feel that both are related to irresponsible attitudes towards healthcare issues?
Yes.
In India, the homeopathic remedy, Arsenicum Album 30C (prepared from arsenic trioxide) is widely prescribed and publicly supplied to adults and children for preventing COVID infections. Inorganic arsenic, known as the “king of poisons” is a highly toxic substance with the potential to cause acute as well as chronic injury to multiple organ systems, mainly skin, lung, liver, and kidneys.
Indian researchers present three cases of acute liver injury, leading to the death of one patient with underlying non-alcoholic steatohepatitis (NASH) cirrhosis, after consumption of the homeopathic remedy AA30 for COVID-19 prevention.
Case one
A 70-year-old man with compensated non-alcoholic steatohepatitis (NASH)-related cirrhosis and diabetes mellitus consumed the homeopathic IB AA30 as prescribed for 12 weeks prior to the onset of symptoms. He presented with jaundice and abdominal distension within four weeks after the onset of loss of appetite and well-being. The patient was not on any other hepatotoxic agents, over-the-counter medications, or herbal and dietary supplements. Investigations revealed the presence of conjugated hyperbilirubinemia, ascites, and abnormal coagulation, suggestive of acute-on-chronic liver failure (ACLF). Further investigations to identify known causes of acute deterioration of underlying cirrhosis were performed, including a transjugular liver biopsy. All competing causes for acute liver injury were meticulously ruled out. These included infections-tests for immunoglobulin M (IgM) for viral hepatitis A and E; hepatitis B surface antigen and IgM antibody to hepatitis B core antigen; nucleic acid tests via polymerase chain reaction for hepatitis C; IgM for herpes zoster and herpes simplex, cytomegalovirus, parvovirus, Epstein-Barr virus. Complete auto-antibody testing for autoimmune hepatitis (AIH) was negative. The Roussel Uclaf Causality Assessment (RUCAM) demonstrated “probable” (score 7) drug-induced liver injury (DILI) and simplified AIH score was less than 5, revealing the cause of acute liver injury leading to ACLF as the homeopathic remedy, AA30. The liver biopsy revealed multiacinar hepatocyte necrosis, lymphocytic, neutrophilic, and eosinophilic inflammation in the absence of interface hepatitis, which were predominantly portal-based in the background of cirrhosis, suggestive of DILI. Analysis of drugs consumed could not be performed in view of inadequate sample availability. The patient and family consented to arsenic analysis in nail and hair samples which revealed extremely high levels of the heavy metal, supportive of arsenic toxicity and associated liver injury in the patient. Evaluation of hair and hair samples of two family members (below detection limits, method detection limit being 0.1 mg/kg), staying in the same household did not reveal levels signifying cluster arsenic poisoning from water or soil sources. The patient succumbed to complications related to ACLF, nine months after the initial diagnosis.
Case two
A 68-year-old male with systemic hypertension controlled on telmisartan who ingested AA30 as prescribed for four weeks prior to the onset of symptoms. There was no associated jaundice or cholestatic symptoms, but liver tests revealed acute hepatitis with an elevation of liver enzymes. The patient was not on any other hepatotoxic agents, over-the-counter medications, or herbal and dietary supplements. Further investigations did not reveal the presence of underlying chronic liver disease or portal hypertension. All competing causes for acute liver injury were meticulously ruled out similar to the extensive workup that was done in case one. The RUCAM demonstrated “probable” (score 8) DILI and simplified AIH score was less than 5, revealing the cause of acute non-icteric hepatitis as the homeopathic remedy, AA30. The liver biopsy revealed perivenular hepatocyte necrosis, with predominantly portal-based mixed cellular inflammation consisting of plasma cells, eosinophils, lymphocytes, and scattered neutrophils. Additionally, ballooning of hepatocytes was marked with scattered rosettes and moderate interphase hepatitis in the presence of mild portal and sinusoidal fibrosis suggestive of DILI. Acute hepatitis resolved after drug withdrawal and finite course of steroids within three months, without any recurrence on follow-up.
Case three
A 48-year-old overweight woman consumed homeopathic AA30 pills as COVID-19 preventive for one week prior to the onset of her symptoms of cholestatic jaundice. Prior to the development of jaundice, she had nonspecific gastrointestinal symptoms such as nausea and progressive loss of appetite. Liver tests revealed conjugated hyperbilirubinemia with highly raised liver enzymes. The patient was not on any other hepatotoxic prescription drugs, over-the-counter medications, or herbal and dietary supplements. Further investigations did not reveal the presence of underlying chronic liver disease or portal hypertension. All competing causes for acute liver injury were meticulously ruled out similar to the extensive workup that was done in case one. The RUCAM demonstrated “probable” (score 7) DILI and simplified AIH score was less than 5, revealing the cause of acute cholestatic hepatitis as the homeopathic remedy, AA30. The liver biopsy revealed spotty, focal hepatocyte necrosis, with predominantly portal-based neutrophilic and eosinophil-rich inflammation, moderate steatosis, and mild interface hepatitis with underlying mild perisinusoidal fibrosis, suggestive of DILI. The acute cholestatic hepatitis resolved after drug withdrawal and a finite course of steroids within six months, without any recurrence on follow-up.
The chemical analysis and toxicology (inductively coupled optical emission spectroscopy and triple-quadrupole gas chromatography with tandem mass spectroscopy method) on two sets of AA30 samples retrieved from case three revealed D-mannose, melezitose, and arsenic respectively, demonstrating batch-to-batch variation due to poor manufacturing practices.
The authors draw the following conclusions: Health regulatory authorities, physicians, general and patient population must be aware of the potential harms associated with the large-scale promotion of untested, alternative medical systems during a medical emergency so as to prevent an “epidemic” of avoidable DILI within the ongoing pandemic. Even though ultra-diluted homeopathic remedies, found ineffective as shown in large-scale meta-analysis, are considered safe for use due to the absence of any active compound beyond 12C dilution. Nonetheless, poor manufacturing practices, use of concentrated tincture formulations, and adulteration and contamination of homeopathic remedies can still pose considerable toxicity in predisposed persons. From a scientific and evidence-based standpoint, it is imperative that the general population and at-risk persons understand that vaccination, and not untested, misleading IBs, remains the best available armamentarium against COVID-19 which helps in fighting back the pandemic.
If you have been following my blog for a while, you probably know the answer to this question. A recent article published in JAMA re-emphasizes it in an exemplary fashion:
According to National Health and Nutrition Examination Survey data, 52% of surveyed US adults reported using at least 1 dietary supplement in the prior 30 days and 31% reported using a multivitamin-mineral supplement. The most commonly cited reason for using supplements is for overall health and wellness and to fill nutrient gaps in the diet. Cardiovascular disease and cancer are the 2 leading causes of death and combined account for approximately half of all deaths in the US annually. Inflammation and oxidative stress have been shown to have a role in both cardiovascular disease and cancer, and dietary supplements may have anti-inflammatory and antioxidative effects.
Objective To update its 2014 recommendation, the US Preventive Services Task Force (USPSTF) commissioned a review of the evidence on the efficacy of supplementation with single nutrients, functionally related nutrient pairs, or multivitamins for reducing the risk of cardiovascular disease, cancer, and mortality in the general adult population, as well as the harms of supplementation.
Population Community-dwelling, nonpregnant adults.
Evidence Assessment The USPSTF concludes with moderate certainty that the harms of beta carotene supplementation outweigh the benefits for the prevention of cardiovascular disease or cancer. The USPSTF also concludes with moderate certainty that there is no net benefit of supplementation with vitamin E for the prevention of cardiovascular disease or cancer. The USPSTF concludes that the evidence is insufficient to determine the balance of benefits and harms of supplementation with multivitamins for the prevention of cardiovascular disease or cancer. Evidence is lacking and the balance of benefits and harms cannot be determined. The USPSTF concludes that the evidence is insufficient to determine the balance of benefits and harms of supplementation with single or paired nutrients (other than beta carotene and vitamin E) for the prevention of cardiovascular disease or cancer. Evidence is lacking and the balance of benefits and harms cannot be determined.
Recommendation The USPSTF recommends against the use of beta carotene or vitamin E supplements for the prevention of cardiovascular disease or cancer. (D recommendation) The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of the use of multivitamin supplements for the prevention of cardiovascular disease or cancer. (I statement) The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of the use of single- or paired-nutrient supplements (other than beta carotene and vitamin E) for the prevention of cardiovascular disease or cancer. (I statement)
The report also elaborates on potential harms:
For many of the vitamins and nutrients reviewed, there was little evidence of serious harms. However, an important harm of increased lung cancer incidence was reported with the use of beta carotene by persons who smoke tobacco or have occupational exposure to asbestos.
Excessive doses of vitamin supplements can cause several known adverse effects; for example, moderate doses of vitamin A supplements may reduce bone mineral density, and high doses may be hepatotoxic or teratogenic. Vitamin D has potential harms, such as a risk of hypercalcemia and kidney stones, when given at high doses. The potential for harm from other supplements at high doses should be carefully considered.
There is nothing new here, of course. I (and others) have been trying to get these points across for many years. But it is nevertheless most gratifying to see the message repeated by a top journal such as JAMA. I hope JAMA is more successful than I was in changing the behavior of the often all too gullible public!
Almost 10 years ago, I posted this:
When I decided to become a doctor I, like most medical students, did so mainly to help suffering individuals. When I became a researcher, I felt more removed from this original ideal. Yet I told myself that, by conducting research, I might eventually contribute to a better health care of tomorrow. Helping suffering patients was still firmly on the agenda. But then I realised that my articles in peer-reviewed medical journals somehow missed an important target: in alternative medicine, one ought to speak not just to health care professionals but also to consumers and patients; after all, it is they who often make the therapeutic decisions in this area.
Once I had realised this, I started addressing the general public by writing for The Guardian and other newspapers, giving public lectures and publishing books for a lay audience, like TRICK OR TREATMENT…The more I did this sort of thing, the more I noticed how important this activity was. And when a friend offered to help me set up a blog, I did not hesitate for long.
So, the reason for my enthusiasm for this blog turns out to be the same as the one that enticed me to go into medicine in the first place. I do believe that it is helpful for consumers to know the truth about alternative medicine. Considering the thousands of sources of daily misinformation in this area, there is an urgent need for well-informed, critical information. By providing it, I am sure I can assist people to make better therapeutic decisions. In a way, I am back where I started all those years ago: hoping to help suffering patients in the most direct way my expertise allows.
Helping vulnerable patients often means warning them from dangerous charlatans, and this is precisely what I frequently try to do with this blog. But how successful are my endeavors?
More often than not, I have no idea and can only hope for the best. Sometimes I do get some feedback that is encouraging and motivates me to carry on. Rarely, however, do I witness immediate, tangible success. And this is why the recent story is so remarkable:
- On 6 June, an Australian acquaintance from the FRIENDS OF SCIENCE IN MEDICINE sent me some material about a planned lecture in the UK by someone promoting dangerous quackery.
- I looked into it and published a blog post about it a few hours later.
- A reader then suggested in the comments section of this post alerting the UK press to it.
- Another reader contacted THE TIMES, and I wrote to several other journalists.
- THE TIMES turned out to be interested in the story.
- They did some research and interviewed Michael Marshall from the GOOD THINKING SOCIETY (and myself).
- Today, THE TIMES published an article about the planned event.
- Finally, a kind person made the article available to those who don’t want to pay for it.
The whole thing amounts to superb teamwork, in my view. It shows how like-minded people who do not even all know each other can manage to achieve a respectable result with little more than goodwill and dedication.
A respectable result?
Of course, the optimal result would be to stop Barbara O’Neill’s UK lectures. Let’s hope this is what eventually will happen – and please let me know if you know more.
This article almost left me speechless:
The back-to-back waves of the COVID-19 pandemic have made a devastating impact globally. The conventional healthcare system is going through serious pressure as cases of the disease continue to spread and the numbers of hospitalizations are increasing every moment. It is becoming hard and challenging because the hospital resources are limited in number as compared with the rate of daily hospitalizations. There are significant shortages of patient care facilities and medical care providers, and on top of that, conventional healthcare systems do not have any proven treatments for COVID-19 patients. Experimental drugs like hydroxychloroquine, followed by remdesivir, ritonavir/lopinavir, and favipiravir are being administered under emergency use authorization (EUA). There is evidence that these experimental medications are causing adverse drug reactions, thus claiming the lives of the hospitalized COVID-19 patients. And those patients who survive the EUA medications and hospitalizations are left with iatrogenic immunosuppressive states leading to increased susceptibility towards secondary life-threatening infections like fungal diseases. In this scenario, complementary and alternative medical systems (CAMS) are providing commendable results with negligible adverse effects or iatrogenic issues in patients with COVID-19. There are several clinical cases recorded and published by various independent homoeopathic doctors and researchers worldwide. But unfortunately, because of a biased medical model and greed for monopolies, these effective treatment methods are not given equal opportunity as their conventional counterparts.
I think the best way to react to this nonsense might be to remind us what the only RCT of homeopathy for COVID showed.
This randomized, double-blind, two-armed, parallel, single-center, placebo-controlled study investigated the effectiveness and safety of the homeopathic medicine, Natrum muriaticum LM2, for mild cases of COVID-19.
Participants aged > 18 years, with influenza-like symptoms and a positive COVID test were recruited and randomized (1:1) into two groups that received different treatments during a period of at-home isolation. One group received the homeopathic medicine Natrum muriaticum, prepared with the second degree of the fifty-millesimal dynamization (LM2; Natrum muriaticum LM2), while the other group received a placebo.
The primary endpoint was time until recovery from COVID-19 influenza-like symptoms. Secondary measures included a survival analysis of the number and severity of COVID-19 symptoms (influenza-like symptoms plus anosmia and ageusia) from a symptom grading scale that was informed by the participant, hospital admissions, and adverse events. Kaplan-Meier curves were used to estimate time-to-event (survival) measures.
Data from 86 participants were analyzed (homeopathy, n = 42; placebo, n = 44). There was no difference in time to recovery between the two groups (homeopathy, n = 41; placebo, n = 41; P = 0.56), nor in a sub-group that had at least 5 moderate to severe influenza-like symptoms at the beginning of monitoring (homeopathy, n = 15; placebo, n = 17; P = 0.06). Secondary outcomes indicated that a 50% reduction in symptom score was achieved significantly earlier in the homeopathy group (homeopathy, n = 24; placebo, n = 25; P = 0.04), among the participants with a basal symptom score ≥ 5. Moreover, values of restricted mean survival time indicated that patients receiving homeopathy might have improved 0.9 days faster during the first five days of follow-up (P = 0.022). Hospitalization rates were 2.4% in the homeopathy group and 6.8% in the placebo group (P = 0.62). Participants reported 3 adverse events in the homeopathy group and 6 in the placebo group.
The authors concluded that the results showed that Natrum muriaticum LM2 was safe to use for COVID-19, but there was no statistically significant difference in the primary endpoints of Natrum muriaticum LM2 and placebo for mild COVID-19 cases.
Another relevant study compared the antibody response of homeopathic and conventional vaccines and placebo in young adults. A placebo-controlled, double-blind RCT was conducted where 150 university students who had received childhood vaccinations were assigned to diphtheria, pertussis, tetanus, mumps, measles homeopathic vaccine, placebo, or conventional diphtheria, pertussis, tetanus (Tdap) and mumps, measles, rubella (MMR) vaccines. The primary outcome was a ≥ two-fold increase in antibodies from baseline following vaccination as measured by ELISA. Participants, investigators, study coordinators, data blood drawers, laboratory technicians, and data analysts were all blinded.
None of the participants in either the homeopathic vaccine or the placebo group showed a ≥ two-fold response to any of the antigens. In contrast, of those vaccinated with Tdap, 68% (33/48) had a ≥ two-fold response to diphtheria, 83% (40/48) to pertussis toxoid, 88% (42/48) to tetanus, and 35% (17/48) of those vaccinated with MMR had a response to measles or mumps antigens (p < 0.001 for each comparison of conventional vaccine to homeopathic vaccine or to placebo). There was a significant increase in geometric mean titres of antibody from baseline for conventional vaccine antigens (p < 0.001 for each), but none for the response to homeopathic antigens or placebo.
The authors concluded that homeopathic vaccines do not evoke antibody responses and produce a response that is similar to placebo. In contrast, conventional vaccines provide a robust antibody response in the majority of those vaccinated.
To give ‘equal opportunity’ to implausible therapies would, in my view, not merely be wrong, it would be scandalously unethical. The role of homeopathy in the prophylaxis and symptomatic management of COVID-19 or other infections is very easily described; it is:
zero,
nil,
nothing,
null,
naught,
zilch.
During their cancer treatment path, cancer patients use numerous drugs,e.g.:
- anticancer medications,
- supportive drugs,
- other prescribed medications,
- herbal remedies,
- other OTC products.
This puts them at risk of significant drug interactions (DIs).
This study describes potential DIs in cancer patients and their prevalence and predictors.
A cross-sectional study was carried out in two centers in the northern West Bank, Palestine. The Lexicomp® Drug Interactions tool (Lexi-Comp, Hudson OH, USA) was applied to check the potential DIs. In addition, the Statistical Package for the Social Sciences (SPSS) was used to show the results and find the associations.
The final analysis included 327 patients. Most of the participants were older than 50 years (61.2%), female (68.5%), and had a solid tumor (74.6%). The total number of potential DIs was 1753, including 1510 drug-drug interactions (DDIs), 24 drug-herb interactions, and 219 drug-food interactions. Importantly, the prevalence of DDIs was 88.1%. In multivariate analysis, the number of potential DDIs significantly decreased with the duration of treatment (p = 0.007), while it increased with the number of comorbidities (p < 0.001) and the number of drugs used (p < 0.001).
The authors concluded that they found a high prevalence of DIs among cancer patients. This required health care providers to develop a comprehensive protocol to monitor and evaluate DIs by improving doctor-pharmacist communication and supporting the role of clinical pharmacists.
What the investigators did not study was the possibility of herb-herb and herb-non-herbal supplement interactions. The reason for this is probably simple: we know too little about these areas to make reasonable judgments. But even in the absence of such considerations, the prevalence of DDIs among cancer patients was high (88.1%). This means that the vast majority of cancer patients had at least one potential DDI. Over half of them were classified as moderately severe or worse.
The lessons seem to be to:
- use only truly necessary drugs and omit all remedies that are of doubtful value,
- educate the public about the risks of interactions,
- be skeptical about the messages of integrative medicine,
- consult a healthcare professional who is competent to make such judgments,
- conduct more rigorous research to increase our knowledge in this complex area.