fraud
Didier Raoult, the French scientist who became well-known for his controversial stance on hydroxychloroquine for treating COVID-19, has featured on this blog before (see here, here, and here). Less well-known is the fact that he has attracted controversy before. In 2006, Raoult and 4 co-authors were banned for one year from publishing in the journals of the American Society for Microbiology (ASM), after a reviewer for Infection and Immunity discovered that four figures from the revised manuscript of a paper about a mouse model for typhus were identical to figures from the originally submitted manuscript, even though they were supposed to represent a different experiment. In response, Raoult “resigned from the editorial board of two other ASM journals, canceled his membership in the American Academy of Microbiology, ASM’s honorific leadership group, and banned his lab from submitting to ASM journals”. In response to Science covering the story in 2012, he stated that, “I did not manage the paper and did not even check the last version”. The paper was subsequently published in a different journal.
Now, the publisher PLOS is marking nearly 50 articles by Didier Raoult, with expressions of concern while it investigates potential research ethics violations in the work. PLOS has been looking into more than 100 articles by Raoult and determined that the issues in 49 of the papers, including reuse of ethics approval reference numbers, warrant expressions of concern while the publisher continues its inquiry.
In August of 2021, Elisabeth Bik wrote on her blog about a series of 17 articles from IHU-Méditerranée Infection that described different studies involving homeless people in Marseille over a decade, but all listed the same institutional ethics approval number. Bik and other commenters on PubPeer have identified ethical concerns in many other papers, including others in large groups of papers with the same ethical approval numbers. Subsequently, Bik has received harassment and legal threats from Raoult.
David Knutson, senior manager of communications for PLOS, sent ‘Retraction Watch’ this statement:
PLOS is issuing interim Expressions of Concerns for 49 articles that are linked to researchers affiliated with IHU-Méditerranée Infection (Marseille, France) and/or the Aix-Marseille University, as part of an ongoing case that involves more than 100 articles in total. Many of the papers in this case include controversial scientist Didier Raoult as a co-author.
Several whistleblowers raised concerns about articles from this institute, including that several ethics approval reference numbers have been reused in many articles. Our investigation, which has been ongoing for more than a year, confirmed ethics approval reuse and also uncovered other issues including:
- highly prolific authorship (a rate that would equate to nearly 1 article every 3 days for one or more individuals), which calls into question whether PLOS’ authorship criteria have been met
- undeclared COIs with pharmaceutical companies
To date, PLOS has completed a detailed initial assessment of 108 articles in total and concluded that 49 warrant an interim Expression of Concern due to the nature of the concerns identified. We’ll be following up with the authors of all articles of concern in accordance with COPE guidance and PLOS policies, but we anticipate it will require at least another year to complete this work.
Raoult is a coauthor on 48 of the 49 papers in question. This summer, Raoult retired as director of IHU-Méditerranée Infection, the hospital and research institution in Marseille that he had overseen since 2011, following an inspection by the French National Agency for the Safety of Medicines and Health Products (ANSM) that found “serious shortcomings and non-compliances with the regulations for research involving the human person” at IHU-Méditerranée Infection and another Marseille hospital. ANSM imposed sanctions on IHU-Méditerranée Infection, including suspending a research study and placing any new research involving people under supervision, and called for a criminal investigation. Other regulators have also urged Marseille’s prosecutor to investigate “serious malfunctions” at the research institution.
Pierre-Edouard Fournier, the new director of IHU-Méditerranée Infection, issued a statement on September 7th that said he had “ensured that all clinical trials in progress relating to research involving the human person (RIPH) were suspended pending the regularization of the situation.” Also in September, the American Society for Microbiology placed expressions of concern on 6 of Raoult’s papers in two of its journals, citing “a ‘scientific misconduct investigation’ by the University of Aix Marseille,” where the researcher also has an affiliation.
___________________________
Christian Lehman predicted on my blog that ” If Covid19 settles in the long-term, he [Raoult] will not be able to escape a minutely detailed autopsy of his statements and his actions. And the result will be devastating.” It seems he was correct.
Max Gerson is well-known to experts in so-called alternative medicine (SCAM). After all, he invented the famous alternative cancer regimen, the Gerson therapy (previously discussed here, here, and here). Not that this treatment works – in fact, it is not just ineffective but also dangerous – but it has prominent promoters, not least King Charles III . As I say, Gerson is well known for his cancer quackery. What hardly anyone knows is that, before he dabbled in cancer, he invented an entirely different medicine.
. As I say, Gerson is well known for his cancer quackery. What hardly anyone knows is that, before he dabbled in cancer, he invented an entirely different medicine.
Max was born as the 3rd of 9 siblings into a Jewish family on October 18, 1881. They lived in Wongrowitz, a part of Poland that at the time belonged to Germany. Max went to school in his hometown and studied medicine in Breslau (Wrocław, now Poland), Wuerzburg, Berlin, and Freiburg. In 1909, he graduated from the University of Freiburg and began practicing medicine at age 28 in Breslau. During WWI, Gerson worked as a surgeon in a military hospital in Breslau and was awarded the ’Iron Cross’ for his service. In 1916, he married Gretchen Hope; the two had three daughters and stayed together until his death.
In 1918, the Gerson family moved to Bielefeld (Germany), and Max specialized in internal medicine as well as neurology. During this period, Gerson developed an anti-inflammatory drug combination and made contact with a local pharmaceutical firm, ‘ASTA Medica’. On the occasion of the firm’s recent 100th jubilee, a German newspaper reported: “The company did business with the well-known Bielefeld physician and inventor Dr. Max Gerson. At the time, he owned the prescription and trademark for a painkiller called Quadronal. Dr. Gerson became a silent partner.”[1] Remarkably, Gerson who published >50 papers (most in German) seems to have no publication on Quadronal.
In his biography of Gerson, Howard Straus (Max’s grandson), explained that Max Gerson did, in fact, develop not just Quadronal for ASTA but also another drug, Quadronox, which however was not as successful as Quadronal. Crucially, Straus makes it very clear that the drug company defrauded Gerson and “never paid a penny to him or his family, nor honored his early ownership of the shares in the company”.[2]
When I was a young clinician in Germany, Quadronal was still quite popular, and I prescribed it regularly. It had been unquestionably the main success for the multi-million firm, ASTA. Today, it is less in use or even no longer available (I am not sure, perhaps someone can fill me in). Gerson’s second drug, Quadronox, seems to have disappeared a long time ago.
I find this story interesting and potentially relevant to the history of Max Gerson. His time in Bielefeld ended when he fled the Nazis (many of his family were killed during the Holocaust). Eventually, Max, his wife, and their three daughters ended up in New York where Gerson tried to establish his anti-cancer regimen. He became fiercely anti-pharma, and many of his followers even claim that he died by being poisoned by the medico-pharmaceutical establishment which allegedly was afraid that his ‘highly successful’ cancer therapy would put them out of business. It is hard to resist the temptation of suspecting a connection between Gerson’s pharma-phobia and the unfair treatment Max received from ASTA in Bielefeld.
Obviously, my knowledge about all this is incomplete, and I would love to hear from people who know more about it.
[1] ASTA-Erfolgsgeschichte startet vor 100 Jahren (westfalen-blatt.de)
[2] Dr. Max Gerson Healing the Hopeless: Amazon.co.uk: Straus, Howard: 9780976018612: Books
Is acupuncture more than a theatrical placebo? Acupuncture fans are convinced that the answer to this question is YES. Perhaps this paper will make them think again.
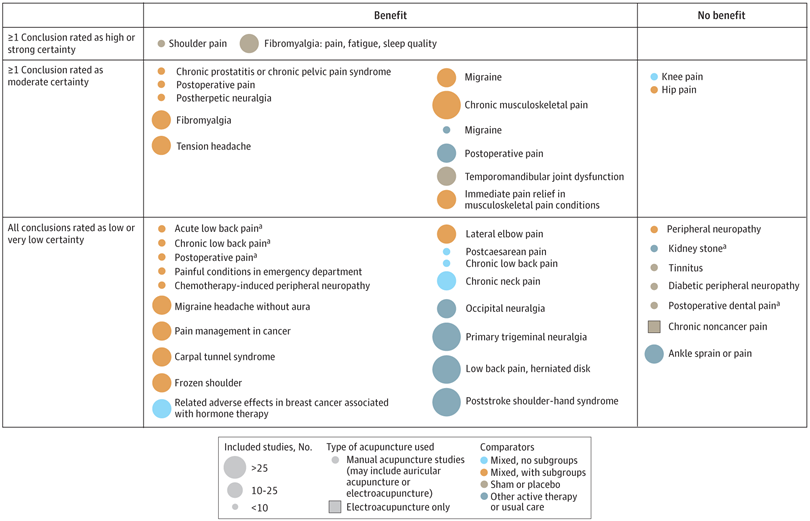
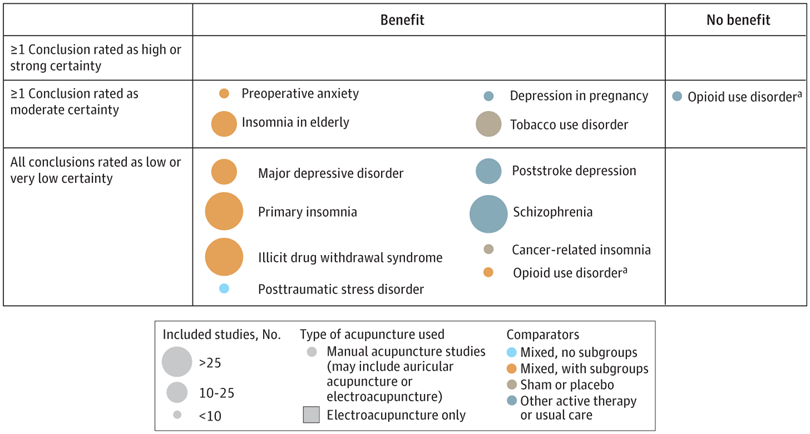
A new analysis mapped the systematic reviews, conclusions, and certainty or quality of evidence for outcomes of acupuncture as a treatment for adult health conditions. Computerized search of PubMed and 4 other databases from 2013 to 2021. Systematic reviews of acupuncture (whole body, auricular, or electroacupuncture) for adult health conditions that formally rated the certainty, quality, or strength of evidence for conclusions. Studies of acupressure, fire acupuncture, laser acupuncture, or traditional Chinese medicine without mention of acupuncture were excluded. Health condition, number of included studies, type of acupuncture, type of comparison group, conclusions, and certainty or quality of evidence. Reviews with at least 1 conclusion rated as high-certainty evidence, reviews with at least 1 conclusion rated as moderate-certainty evidence and reviews with all conclusions rated as low- or very low-certainty evidence; full list of all conclusions and certainty of evidence.
A total of 434 systematic reviews of acupuncture for adult health conditions were found; of these, 127 reviews used a formal method to rate the certainty or quality of evidence of their conclusions, and 82 reviews were mapped, covering 56 health conditions. Across these, there were 4 conclusions that were rated as high-certainty evidence and 31 conclusions that were rated as moderate-certainty evidence. All remaining conclusions (>60) were rated as low- or very low-certainty evidence. Approximately 10% of conclusions rated as high or moderate-certainty were that acupuncture was no better than the comparator treatment, and approximately 75% of high- or moderate-certainty evidence conclusions were about acupuncture compared with a sham or no treatment.
Three evidence maps (pain, mental conditions, and other conditions) are shown below
The authors concluded that despite a vast number of randomized trials, systematic reviews of acupuncture for adult health conditions have rated only a minority of conclusions as high- or moderate-certainty evidence, and most of these were about comparisons with sham treatment or had conclusions of no benefit of acupuncture. Conclusions with moderate or high-certainty evidence that acupuncture is superior to other active therapies were rare.
These findings are sobering for those who had hoped that acupuncture might be effective for a range of conditions. Despite the fact that, during recent years, there have been numerous systematic reviews, the evidence remains negative or flimsy. As 34 reviews originate from China, and as we know about the notorious unreliability of Chinese acupuncture research, this overall result is probably even more negative than the authors make it out to be.
Considering such findings, some people (including the authors of this analysis) feel that we now need more and better acupuncture trials. Yet I wonder whether this is the right approach. Would it not be better to call it a day, concede that acupuncture generates no or only relatively minor effects, and focus our efforts on more promising subjects?
An international team of researchers described retracted papers originating from paper mills, including their characteristics, visibility, and impact over time, and the journals in which they were published. The term paper mill refers to for-profit organizations that engage in the large-scale production and sale of papers to researchers, academics, and students who wish to, or have to, publish in peer-reviewed journals. Many paper mill papers included fabricated data.
All paper mill papers retracted from 1 January 2004 to 26 June 2022 were included in the study. Papers bearing an expression of concern were excluded. Descriptive statistics were used to characterize the sample and analyze the trend of retracted paper mill papers over time, and to analyze their impact and visibility by reference to the number of citations received.
In total, 1182 retracted paper mill papers were identified. The publication of the first paper mill paper was in 2004 and the first retraction was in 2016; by 2021, paper mill retractions accounted for 772 (21.8%) of the 3544 total retractions. Overall, retracted paper mill papers were mostly published in journals of the second highest Journal Citation Reports quartile for impact factor (n=529 (44.8%)) and listed four to six authors (n=602 (50.9%)). Of the 1182 papers, almost all listed authors of 1143 (96.8%) paper mill retractions came from Chinese institutions, and 909 (76.9%) listed a hospital as a primary affiliation. 15 journals accounted for 812 (68.7%) of 1182 paper mill retractions, with one journal accounting for 166 (14.0%). Nearly all (n=1083, 93.8%) paper mill retractions had received at least one citation since publication, with a median of 11 (interquartile range 5-22) citations received.
The authors concluded that papers retracted originating from paper mills are increasing in frequency, posing a problem for the research community. Retracted paper mill papers most commonly originated from China and were published in a small number of journals. Nevertheless, detected paper mill papers might be substantially different from those that are not detected. New mechanisms are needed to identify and avoid this relatively new type of misconduct.
China encourages its researchers to publish papers in return for money and career promotions. Furthermore, medical students at Chinese universities are required to produce a scientific paper in order to graduate. Paper mills openly advertise their services on the Internet and maintain a presence on university campuses. The authors of this analysis reference another recent article (authored by two Chinese researchers) that throws more light on the problem:
This study used data from the Retraction Watch website and from published reports on retractions and paper mills to summarize key features of research misconduct in China. Compared with publicized cases of falsified or fabricated data by authors from other countries of the world, the number of Chinese academics exposed for research misconduct has increased dramatically in recent years. Chinese authors do not have to generate fake data or fake peer reviews for themselves because paper mills in China will do the work for them for a price. Major retractions of articles by authors from China were all announced by international publishers. In contrast, there are few reports of retractions announced by China’s domestic publishers. China’s publication requirements for physicians seeking promotions and its leniency toward research misconduct are two major factors promoting the boom of paper mills in China.
As the authors of the new analysis point out: “Fraudulent papers have negative consequences for the scientific community and the general public, engendering distrust in science, false claims of drug or device efficacy, and unjustified academic promotion, among other problems.” On this blog, I have often warned of research originating from China (some might even think that this is becoming an obsession of mine but I do truly think that this is very important). While such fraudulent papers may have a relatively small impact in many areas of healthcare, their influence in the realm of TCM (where the majority of research comes from China) is considerable. In other words, TCM research is infested by fraud to a degree that prevents drawing meaningful conclusions about the value of TCM treatments.
I feel strongly that it is high time for us to do something about this precarious situation. Otherwise, I fear that in the near future no respectable scientist will take TCM seriously.
It has been reported that a naturopath from the US who sold fake COVID-19 immunization treatments and fraudulent vaccination cards during the height of the coronavirus pandemic has been sentenced to nearly three years in prison. Juli A. Mazi pleaded guilty last April in federal court in San Francisco to one count of wire fraud and one count of false statements related to health care matters. Now District Judge Charles R. Breyer handed down a sentence of 33 months, according to Joshua Stueve, a spokesperson for the U.S. Department of Justice. Mazi, of Napa, was ordered to surrender to the Bureau of Prisons on or before January 6, 2023.
The case is the first federal criminal fraud prosecution related to fraudulent Centers for Disease Control and Prevention vaccination cards for COVID-19, according to the U.S. Department of Justice. In August, Breyer denied Mazi’s motion to withdraw her plea agreement after she challenged the very laws that led to her prosecution. Mazi, who fired her attorneys and ended up representing herself, last week filed a letter with the court claiming sovereign immunity. Mazi said that as a Native American she is “immune to legal action.”
She provided fake CDC vaccination cards for COVID-19 to at least 200 people with instructions on how to complete the cards to make them look like they had received a Moderna vaccine, federal prosecutors said. She also sold homeopathic pellets she fraudulently claimed would provide “lifelong immunity to COVID-19.” She told customers that the pellets contained small amounts of the virus and would create an antibody response. Mazi also offered the pellets in place of childhood vaccinations required for attendance at school and sold at least 100 fake immunization cards that said the children had been vaccinated, knowing the documents would be submitted to schools, officials said. Federal officials opened an investigation against Mazi after receiving a complaint in April 2021 to the Department of Health and Human Services Office of Inspector General hotline.
_______________________
On her website, Mazi states this about herself:
Juli Mazi received her doctorate in Naturopathic Medicine from the National University of Natural Medicine in Portland, Oregon where she trained in the traditional medical sciences as well as ancient and modern modalities that rely on the restorative power of Nature to heal. Juli Mazi radiates the vibrant health she is committed to helping her patients achieve. Juli’s positive outlook inspires confidence; her deep well of calm puts people at immediate ease. The second thing they notice is that truly she listens. Dr. Mazi’s very presence is healing.
On this site, she also advocates all sorts of treatments and ideas which I would call more than a little strange, for instance, coffee enemas:
Using a coffee enema is a time-tested remedy for detoxification, but it is not without risks. If you are not careful, the process can cause internal burns. In addition, improperly brewed coffee can lead to electrolyte imbalances and dehydration, and coffee enemas are not recommended for pregnant women or young children.
To make coffee enemas safe and effective, always choose quality organic coffee. A coffee enema should be free of toxins and pesticides. Use a reusable enema kit with stainless steel or silicone hosing for safety. Moreover, do not use a soft plastic or latex enema bags. It is also essential to limit the length of time that the coffee spends in the container.
A coffee enema should be held for 12 to 15 minutes and then released in the toilet. You may repeat the process as necessary. Usually, the procedure should be done once or twice a day. However, if you are experiencing acute toxicity, you can use a coffee enema as often as needed. Make sure you have had a bowel movement before making the coffee enema. Otherwise, the process may be hindered.
Perhaps the most interesting thing on her website is her advertisement of the fact that her peers not just tolerate such eccentricities but gave Mazi an award for ‘BEST ALTERNATIVE HEALTH & BEST GENERAL PRACTITIONER’.
To me, this suggests that US ‘doctors of naturopathy’ and their professional organizations live on a different planet, a planet where evidence counts for nothing and dangerously misleading patients seems to be the norm.
I know, I have often posted nasty things about integrative medicine and those who promote it. Today, I want to make good for all my sins and look at the bright side.
Imagine you are a person convinced of the good that comes from so-called alternative medicine (SCAM). Imagine you believe it has stood the test of time, is natural, holistic, tackles the root problems of illness, etc., etc. Imagine you are such a person.
Your convictions made you support more research into SCAM because you feel that evidence is needed for it to be more generally accepted. So, you are keen to see more studies proving the efficacy of this or that SCAM in the management of this or that condition.
This, unfortunately, is where the problems start.
Not only is there not a lot of money and even fewer scientists to do this research, but the amount of studies that would need doing is monstrously big:
- There are hundreds of different types of SCAM.
- Each SCAM is advocated for hundreds of conditions.
Consequently, tens of thousands of studies are needed to only have one trial for each specific research question. This is tough for a SCAM enthusiast! It means he/she has to wait decades to see the light at the end of the tunnel.
But then it gets worse – much worse!
As the results of these studies come in, one after the other, you realize that most of them are not at all what you have been counting on. Many can be criticized for being of dismal quality and therefore inconclusive, and those that are rigorous tend to be negative.
Bloody hell! There you have been waiting patiently for decades and now you must realize that this wait did not take you anywhere near the goal that was so clear in your sight. Most reasonable people would give up at this stage; they would conclude that SCAM is a pipedream and direct their attention to something else. But not you! You are single-minded and convinced that SCAM is the future. Some people might even call you obsessed – obsessed and desperate.
It is out of this sense of desperation that the idea of integrative medicine was born. It is a brilliant coup that solves most of the insurmountable problems outlined above. All you need to do is to take the few positive findings that did emerge from the previous decades of research, find a political platform, and loudly proclaim:
SCAM does work.
Consumers like SCAM.
SCAM must be made available to all.
Consumers deserve the best of both worlds.
The future of healthcare evidently lies in integrated medicine.
Forgotten are all those irritating questions about the efficacy of this or that treatment. Now, it’s all about the big issue of wholesale integration of SCAM. Forgotten is the need for evidence – after all, we had decades of that! – now, the issue is no longer scientific, it is political.
And if anyone has the audacity to ask about evidence, he/she can be branded as a boring nit-picker. And if anyone doubts the value of integrated medicine, he/she will be identified as a politically incorrect dinosaur.
Mission accomplished!
Electroacupuncture (EA) is often advocated for depression and sleep disorders but its efficacy remains uncertain. The aim of this study was, therefore, to “assess the efficacy and safety of EA as an alternative therapy in improving sleep quality and mental state for patients with insomnia and depression.”
A 32-week patient- and assessor-blinded, randomized, sham-controlled clinical trial (8-week intervention plus 24-week follow-up) was conducted from September 1, 2016, to July 30, 2019, at 3 tertiary hospitals in Shanghai, China. Patients were randomized to receive
- EA treatment and standard care,
- sham acupuncture (SA) treatment and standard care,
- standard care only as control.
Patients in the EA or SA groups received a 30-minute treatment 3 times per week (usually every other day except Sunday) for 8 consecutive weeks. All treatments were performed by licensed acupuncturists with at least 5 years of clinical experience. A total of 6 acupuncturists (2 at each center; including X.Y. and S.Z.) performed EA and SA, and they received standardized training on the intervention method before the trial. The regular acupuncture method was applied at the Baihui (GV20), Shenting (GV24), Yintang (GV29), Anmian (EX-HN22), Shenmen (HT7), Neiguan (PC6), and SanYinjiao (SP6) acupuncture points, with 0.25 × 25-mm and 0.30 × 40-mm real needles (Wuxi Jiajian Medical Device Co, Ltd), or 0.30 × 30-mm sham needles (Streitberger sham device [Asia-med GmbH]).
For patients in the EA group, rotating or lifting-thrusting manipulation was applied for deqi sensation after needle insertion. The 2 electrodes of the electrostimulator (CMNS6-1 [Wuxi Jiajian Medical Device Co, Ltd]) were connected to the needles at GV20 and GV29, delivering a continuous wave based on the patient’s tolerance. Patients in the SA group felt a pricking sensation when the blunt needle tip touched the skin, but without needle insertion. All indicators of the nearby electrostimulator were set to 0, with the light switched on. Standard care (also known as treatment as usual or routine care) was used in the control group. Patients receiving standard care were recommended by the researchers to get regular exercise, eat a healthy diet, and manage their stress level during the trial. They were asked to keep the regular administration of antidepressants, sedatives, or hypnotics as well. Psychiatrists in the Shanghai Mental Health Center (including X.L.) guided all patients’ standard care treatment and provided professional advice when a patient’s condition changed.
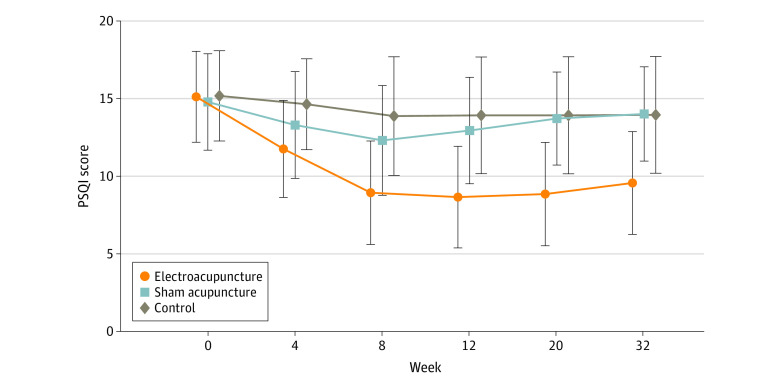
The primary outcome was change in Pittsburgh Sleep Quality Index (PSQI) from baseline to week 8. Secondary outcomes included PSQI at 12, 20, and 32 weeks of follow-up; sleep parameters recorded in actigraphy; Insomnia Severity Index; 17-item Hamilton Depression Rating Scale score; and Self-rating Anxiety Scale score.
Among the 270 patients (194 women [71.9%] and 76 men [28.1%]; mean [SD] age, 50.3 [14.2] years) included in the intention-to-treat analysis, 247 (91.5%) completed all outcome measurements at week 32, and 23 (8.5%) dropped out of the trial. The mean difference in PSQI from baseline to week 8 within the EA group was -6.2 (95% CI, -6.9 to -5.6). At week 8, the difference in PSQI score was -3.6 (95% CI, -4.4 to -2.8; P < .001) between the EA and SA groups and -5.1 (95% CI, -6.0 to -4.2; P < .001) between the EA and control groups. The efficacy of EA in treating insomnia was sustained during the 24-week postintervention follow-up. Significant improvement in the 17-item Hamilton Depression Rating Scale (-10.7 [95% CI, -11.8 to -9.7]), Insomnia Severity Index (-7.6 [95% CI, -8.5 to -6.7]), and Self-rating Anxiety Scale (-2.9 [95% CI, -4.1 to -1.7]) scores and the total sleep time recorded in the actigraphy (29.1 [95% CI, 21.5-36.7] minutes) was observed in the EA group during the 8-week intervention period (P < .001 for all). No between-group differences were found in the frequency of sleep awakenings. No serious adverse events were reported.
The result of the blinding assessment showed that 56 patients (62.2%) in the SA group guessed wrongly about their group assignment (Bang blinding index, −0.4 [95% CI, −0.6 to −0.3]), whereas 15 (16.7%) in the EA group also guessed wrongly (Bang blinding index, 0.5 [95% CI, 0.4-0.7]). This indicated a relatively higher degree of blinding in the SA group.
The authors concluded that, in this randomized clinical trial of EA treatment for insomnia in patients with depression, quality of sleep improved significantly in the EA group compared with the SA or control group at week 8 and was sustained at week 32.
This trial seems rigorous, it has a sizable sample size, uses a credible placebo procedure, and is reported in sufficient detail. Why then am I skeptical?
- Perhaps because we have often discussed how untrustworthy acupuncture studies from China are?
- Perhaps because I fail to see a plausible mechanism of action?
- Perhaps because the acupuncturists could not be blinded and thus might have influenced the outcome?
- Perhaps because the effects of sham acupuncture seem unreasonably small?
- Perhaps because I cannot be sure whether the acupuncture or the electrical current is supposed to have caused the effects?
- Perhaps because the authors of the study are from institutions such as the Shanghai Municipal Hospital of Traditional Chinese Medicine, the Department of Acupuncture and Moxibustion, Huadong Hospital, Fudan University, Shanghai,
- Perhaps because the results seem too good to be true?
If you have other and better reasons, I’d be most interested to hear them.
The AMA has recently published a short article that – even though not addressing so-called alternative medicine (SCAM) directly – has considerable relevance for the field:
It’s increasingly common for patients to encounter nonphysician practitioners as members of their health care teams. Meanwhile, ever more nonphysician practitioners have received advanced training resulting in a doctorate degree, such as the doctor of nursing practice.
To help patients keep pace with these changes, physicians should make new strides to clarify their roles and credentials vis-a-vis other members of the health care team and also promote collaboration among all health professionals, according to an AMA Council on Ethical and Judicial Affairs report that was adopted at the 2022 AMA Interim Meeting.
The core issue is that “the skill sets and experience of nonphysician practitioners are not the same as those of physicians.” Thus, when nonphysician practitioners identify themselves as “doctors”—consistent with the doctoral-level degrees they earned—“it may create confusion and be misleading to patients and other practitioners,” says the report.
In fact, surveys (PDF) performed as part of the AMA Truth in Advertising Campaign have found that while patients strongly support physician-led health care teams, many are confused about the level of education and training of health professionals—and the confusion isn’t limited to nonphysician practitioners who hold doctorates. For example, roughly one-fifth of respondents think psychiatrists are not physicians, while a similar number think nurse practitioners are physicians.
The AMA Code of Medical Ethics touches on this issue in an opinion on collaborative care, which provides guidance on the roles of physicians in team-based settings where a mix of health professionals provide care.
In SCAM, we have the problem that practitioners often call themselves doctors or physicians without having a medical degree. This confuses patients who might consult and trust these practitioners assuming they have studied medicine. We recently discussed the case of a naturopath who called himself a doctor and failed to diagnose a rectal tumor of his patient. Much more dramatic was the case of a UK-based chiropractor who called herself a doctor, thus attracting a patient suffering from complex health issues contraindicating spinal manipulations. She nonetheless manipulated his neck and promptly killed him.
I know that patients are being misled every day by SCAM practitioners (ab)using the ‘Dr.’ title. Therefore, the AMA reminder is an important, timely, and necessary lesson for SCAM. I feel that the professional organizations of SCAM providers should issue similar reminders to their members and make sure they behave appropriately.
The U.S. Food and Drug Administration issued warning letters to seven companies for illegally selling dietary supplements that claim to cure, treat, mitigate or prevent cardiovascular disease or related conditions, such as atherosclerosis, stroke or heart failure, in violation of the Federal Food, Drug, and Cosmetic Act (FD&C Act). The FDA is urging consumers not to use these or similar products because they have not been evaluated by the FDA to be safe or effective for their intended use and may be harmful.
The warning letters were issued to:
- Essential Elements (Scale Media Inc.);
- Calroy Health Sciences LLC;
- Iwi;
- BergaMet North America LLC;
- Healthy Trends Worldwide LLC (Golden After 50);
- Chambers’ Apothecary;
- Anabolic Laboratories, LLC.
“Given that cardiovascular disease is the leading cause of death in the U.S., it’s important that the FDA protect the public from products and companies that make unlawful claims to treat it. Dietary supplements that claim to cure, treat, mitigate or prevent cardiovascular disease and related conditions could potentially harm consumers who use these products instead of seeking safe and effective FDA-approved treatments from qualified health care providers,” said Cara Welch, Ph.D., director of the Office of Dietary Supplement Programs in the FDA’s Center for Food Safety and Applied Nutrition. “We encourage consumers to remain vigilant when shopping online or in stores to avoid purchasing products that could put their health at risk.”
Under the FD&C Act, products intended to diagnose, cure, treat, mitigate or prevent disease are drugs and are subject to the requirements that apply to drugs, even if they are labeled as dietary supplements. Unlike drugs approved by the FDA, the agency has not evaluated whether the unapproved products subject to the warning letters announced today are effective for their intended use, what the proper dosage might be, how they could interact with FDA-approved drugs or other substances, or whether they have dangerous side effects or other safety concerns.
The FDA advises consumers to talk to their doctor, pharmacist or other health care provider before deciding to purchase or use any dietary supplement or drug. Some supplements might interact with medicines or other supplements. Health care providers will work with patients to determine which treatment is the best option for their condition.
If a consumer thinks that a product might have caused a reaction or an illness, they should immediately stop using the product and contact their health care provider. The FDA encourages health care providers and consumers to report any adverse reactions associated with FDA-regulated products to the agency using MedWatch or the Safety Reporting Portal.
The FDA has requested responses from the companies within 15 working days stating how they will address the issues described in the warning letters or provide their reasoning and supporting information as to why they think the products are not in violation of the law. Failure to correct violations promptly may result in legal action, including product seizure and/or injunction.
Recently, I received an email with this ‘special offer’ for purchasing a book and was impressed – but not in a positive sense:
Dr Farokh’s commendable work at upto 22% off – Healing Cancer. For Limited time period only.
Healing Cancer: A Homoeopathic Approach
As a homeopath one should not deter oneself in dealing with any type of cases, be it cancer. But for executing that an ultimate guidance is needed. Cancer is so much prevalent and challenging medical problem of today that a trustworthy source of accurate information becomes pertinent and this work of Dr. Farokh Master immediately propels at the top of quality books for cancer. Based on Master’s 40 years of experience this book was written for students to understand the basis of oncology and for practitioners for brushing-up of their knowledge in this growing discipline. Author says that to get a grasp on cancer cases we should believe in the potential of the homeopathic treatment, that healing from cancer refers to internal process of becoming whole and feeling harmonious with yourself and your environment.To even start with handling the cases of cancer one should be aware of understanding of cancer, its cause, pathophysiology, different types, conventional treatment and their side effects, integrative medicines, social problems in the treatment, such topics are well casted by Volume 1 of the book.
Peak points of Volume 1- • A full chapter is dealing with Iscador, a relatively old method, very effective but unfortunately underemployed.• Published papers about Homeopathy in the treatment of cancer are presented before the last chapter which is on some of most used allopathic drugs in cancer with a focus on their side-effects. After the coverage of basic information on oncology in Volume 1 comes the Volume 2 which explores topics like understanding cancer from homoeopathic point of view, constitutional remedies, therapeutics of individual cancers, nutrition, general management.
Peak points of Volume 2-• A whole chapter on Cadmium salts and cancer.• 51 “lesser known remedies” are briefly quoted and their usefulness in different situations and types of cancer exposed.• A long chapter deals with the “Indian drugs”, it is important that these remedies are used mostly in tincture or low potencies, as herbal or Ayurvedic remedies or food supplements relieving the patients. • The choice and differentiation between the remedies is then helped by the “Repertory of Cancer”, very well compiled and a highly useful section. “Clinical tips from my practice” given as a sub-chapter. • It ends with recommendations on how to deal with radiation illness and the side-effects of conventional treatment, as well as the treatment of pain and help with palliative care.
For fighting and curing cancer and improving the quality and quantity of life of people, knowledge of Homeopathy, both philosophically and scientifically is needed which this work of art portrays delightfully.
About Book Author:
Dr. Farokh J. Master’s birth into homeopathy was in the year 1976, when he joined Bombay homeopathic medical college, after giving up his studies at the orthodox school of medicine. Dr Master was instrumental in starting homeopathic out-patient dept in many allopathic hospitals viz. Bombay Hospital, KEM Hospital & Ruby Hall, Pune. Besides his work as a senior Homeopath of the HHC, Dr. Farokh Master is teaching homeopathy (advanced level) at the Mumbai Homeopathic Medical College, part of Mumbai university. He is also teaching at other homeopathic colleges in India and abroad. He has given seminars in various countries like Austria, Australia, India, Japan etc. Dr Master has written more than 50 books like -The Homeopathic Dream Dictionary, Cross References of the Mind, Perceiving Rubrics of the Mind, The State of Mind affecting the Foetus, Tumors and Homeopathy, The Bedside Organon of Medicine, The proving of Mocassin Snake, Bungarus, etc. Dr. Master is the originator of many recent new approaches and insights in homoeopathy.
Some people claim that homeopaths are not dangerous and argue that their placebos cannot harm patients. I have long disagreed with this position. As homeopathy is not an effective therapy (it has no effects beyond placebo), its use simply means allowing diseases to remain untreated.
- If we are dealing with a common cold, this might be little more than a costly nuisance.
- If we are dealing with a chronic condition such as arthritis, it means causing unnecessary suffering.
- If we are dealing with life-threatening diseases like cancer, it means shortening the life of patients.
This is the politest way I can put it. There are of course other, less polite terms for ‘shortening a life’! Most of us shy away from using them in the context of homeopathy. In the case of the author of this book, we might make an exception. In my view, he is someone who is deluded to the point where he is ready to kill his patients with homeopathy.
PS
Iscador is not even a homeopathic remedy.