critical thinking
A 2020 review entitled ‘Prevention, Treatment and Management of Tuberculosis through Combinational Approaches of Different Indian Systems of Medicine’ stated that recent research suggests that Homeopathic treatment along with the antibiotics synergise the effect of antibiotics while reaching to its site of action. This is a surprising finding, if there ever was one. Therefore, I looked a bit closer with a view of determining what the original data were that led to this conclusion.
Here is the unaltered section on homeopathy from the review:
Homeopathy is a special, organic, holistic medicine process which activates the healing responses of the body without any known contraindications or common side effects. In his book Organon of Medicine, aka Organon of Healing Art, the founder of Homeopathy, Samuel Hahnemann, developed these principles. Homoeopathy does not have holistic treatment and hence is incapable of curing TB because of homeopathic portions of water, which is not enough to treat TB, but recent research suggests the use of homeopathic treatment along with the antibiotics, which results in synergising the effect of antibiotics while reaching to its site of action. As standard MDR-TB medicines are second-generation antibiotics taken for 24–27 months, thus, apart from conventional treatment, homeopathy in MDR-TB tends to improve outcome. Hence, it increases the bioavailability of the isoniazid and rifampicin, which resulted in the reduction of tuberculosis therapy. This study has been proving by analysing following parameters as below:
There was no significant difference in smear conversion from positive to negative at 95 % CI between homeopathy (H) and individualised standard treatment regimen (SR) + placebo (P). However, the conversion culture conversion from positive to negative in SR + H was seen in 29 (48.3 %) patients and 23 (38.3 %) patients in SR + P group (p = 0.269) (ITT) and 27 (55.1 %); 21 (42.8 %), p = 0.225 (PP) implying that as compared to SR + P group, culture conversion in SR + H group was more by 10 %. So the difference, although favourable to the SR + H group is not statistically significant as shown in table 4.
All patients had far advanced lung disease as evident from the extensive infiltration, cavitation ns and fibrosis/collapse. Chest X-ray (CXR) were further assessed using RAT (Radiological Assessment Tool), examples of one case rated as + 5 and one case as _4. Significant improvements were seen statistically in CXR in the SR + H 37 (61.7 %); as compared to SR + P 20 (33.3 %), p = 0.002 at 95 % CI (ITT). Homeopathy system having all possibility to enhance the treatment and management of the TB [13].
To be clear: there is no further mention or discussion of homeopathy in this paper. The above-quoted section (its multiple falsehoods are so obvious that I probably don’t need to mention them here) is thus the only evidence provided for backing up the claim that Homeopathic treatment along with the antibiotics synergise the effect of antibiotics while reaching to its site of action. I fail to see how the information provided supports the claim. Moreover, I am not aware of any sound evidence that would support the claim that homeopathy acts synergistically to antibiotics.
In the reference list, I found one of my own articles cited as reference 13 (see above). It refers to this article: Ernst E. A systematic review of systematic reviews of homeopathy. Dr J Clin Pharmacol 2002; 54: 577–582. Here is its abstract:
Homeopathy remains one of the most controversial subjects in therapeutics. This article is an attempt to clarify its effectiveness based on recent systematic reviews. Electronic databases were searched for systematic reviews/meta-analysis on the subject. Seventeen articles fulfilled the inclusion/exclusion criteria. Six of them related to re-analyses of one landmark meta-analysis. Collectively they implied that the overall positive result of this meta-analysis is not supported by a critical analysis of the data. Eleven independent systematic reviews were located. Collectively they failed to provide strong evidence in favour of homeopathy. In particular, there was no condition which responds convincingly better to homeopathic treatment than to placebo or other control interventions. Similarly, there was no homeopathic remedy that was demonstrated to yield clinical effects that are convincingly different from placebo. It is concluded that the best clinical evidence for homeopathy available to date does not warrant positive recommendations for its use in clinical practice.
I fail to see how my paper backs up the very odd sentence: Homeopathy system having all possibility to enhance the treatment and management of the TB.
This is much more than sloppiness. In fact, it is one of the clearest cases of scientific misconduct I have ever come across. What is more, if its message would get adopted, it has the potential to do considerable harm. The authors of the paper in question, Priyanka Sharma , Ramesh K. Goyal, and Mukesh Nandave, declared no conflicts of interest; their affiliation is provided as ‘Departments of Pharmacology and Toxicology, Delhi Pharmaceutical Sciences and Research University, New Delhi, India’. The journal that published their review is ‘Drug Research‘, a publication which I always had thought was reputable.
I am not sure what I should do next.
Any advice?
‘HOMEOPATHY360’ are fiercely decided to defend homeopathy, no matter what. They state that we promise to stand by your side always to fight against the critical attacks on Homeopathy… Therefore, I was not really surprised when, a couple of days ago, I received an email by them urging me to support US homeopaths against the threat by the FDA. Here is part of this correspondence:
… If you want to know more about the FDA’s proposed new rules for homeopathic medicines, here’s a summary of the most important points:
- The new rules, if adopted, will allow the FDA to withdraw even properly manufactured and labeled homeopathic medicines from the marketplace. This is puzzling because these have never posed any sort of safety concern according to an initial review of public FDA records by Americans for Homeopathy Choice.
- It is clear that the FDA intends to use this authority and has even mentioned specific medicines such as Belladonna, Nux vomica and Lachesis muta in its public statements regarding enforcement.
- The authority for this kind of assault on homeopathy will result from the declaration by the FDA that all homeopathic medicines are “new drugs.” We all know this is nonsense. Homeopathic medicines have been around for 200 years.
- But this nonsense declaration means that under U.S. law all homeopathic remedies will become technically “illegal” and subject to withdrawal from the marketplace. If the FDA just thinks there is a problem with a homeopathic medicine, it can withdraw it forever without conducting any sort of investigation.
- Since the agency has already said that it thinks that Belladonna, Nux vomica, Lachesis muta and several other remedies are dangerous, we can anticipate that it will try to remove them from the marketplace as soon as its new rules are adopted.
- But, it won’t be possible for Americans to get remedies that are banned sent to them from abroad. The FDA will simply stop these remedies at the border.
I could tell you more, but what I’ve told you so far should convince you that we ought to help the American homeopathy community defeat these unreasonable and misinformed rules. The rules simply do not reflect the realities of homeopathic medicines, namely, that they are nontoxic, mild, effective and have few, if any, side-effects. And, homeopaths use them in ways that individualize treatment. That this is the best way to treat patients was discovered by Samuel Hahnemann 200 years ago.
The enemies of homeopathy are everywhere and they appear to be stepping up their attacks. That’s why the world homeopathy community must work together to stand up to them…
_________________________________________________________________
I have reported about the FDA initiatives on homeopathy before. In 2015, they started it with a public hearing. Since then, the FDA also issued several warnings to manufacturers who were putting consumers at risk (see, for instance, here, here, and here).
What the FDA seem to be trying to do is nothing else but meeting their ethical, moral and legal responsibility vis a vis consumer safety. Homeopathy has had a free ride for far too long. It is high time that this sector joins the 21st century.
The above quote, with its bonanza of bogus claims and falsehoods, shows the urgency of this task. The defenders of homeopathy seem to live on a different planet where rationality, facts and evidence can easily be over-ruled by creed, dogma and wishful thinking. If homeopaths want their trade to join the realm of real medicine they need, at the very minimum, to show with sound evidence:
- that their remedies generate more good than harm,
- that they adhere to acceptable quality standards.
Failing this – and so far, homeopaths not only failed at this task but continue bombarding us with an incessant flow of bogus and dangerous claims – homeopathics cannot be considered to be medicines, and homeopaths cannot be called responsible healthcare professionals. It is high time to stop turning a blind eye to the double standards that have been applied for 200 years.
Neurolinguistic programming (NLP) was developed in the mid-seventies. It is a so-called alternative therapy (SCAM) that is not easy to define. Those who started it and those involved in it use such vague language that NLP means different things to different people. One metaphor keeps recurring: NLP claims to help people change by teaching them to program their brains. We were given brains, we are told, without an instruction manual, and NLP offers a user-manual for the brain. Consciously or unconsciously, NLP is based on the assumptions that:
- the unconscious mind constantly influences our conscious thoughts and actions;
- Freud’s theories are correct;
- hypnotherapy is effective.

Wikipedia is more outspoken about it: Neuro-linguistic programming (NLP) is a pseudoscientific approach to communication, personal development, and psychotherapy…
Despite the fact that NLP is unproven (to say the least), the COLLEGE OF MEDICINE AND INTEGRATED HEALTH (COMAIH) is sufficiently impressed by NLP to offer a course for GPs and SCAM practitioners. Here is their announcement:
Neurolinguistic Healthcare in association with the College of Medicine brings you a 2-day Introduction to Hypnosis, Neurolinguistic Programming (NLP) and Neurolinguistic Healthcare (NLH). Dr Wong and Dr Akhtar who lead the course are Trainers in NLP and Hypnosis and GPs who apply their skills in daily practice within the 10-minute consultation. The course is suitable for both medical professionals and complementary therapists. This is a limited training event offered by them to share their years of knowledge and skills with you.
You will learn:
-
- A basic overview of NLP and the most useful aspects to use it to begin making effective changes in how you and the people you treat think and behave
- An understanding of the NLH model of the mind so that you understand the concepts of therapy using this mixed hypnosis/ NLP approach in relation to health.
- The ability to Hypnotise effectively in a very short period of time with practical experience – the ability to go through all the stages of hypnosis – the induction, deepening, therapy, and emergence, including rapid hypnosis techniques. (Hypnosis courses which are less practical often charge in excess of £2000 for this)
- Learn at least 3 therapeutic techniques, including the NLP therapeutic techniques which work much better in trance, so using and applying the skills you will learn in hypnosis
- Access to an online mentorship programme with Dr Akhtar or Dr Wong for 6 months and who will provide 3x30mins group webinar meetings after the course to ensure any remaining questions get answered and that you are actually going forth to apply these skills. (worth another £600 in value)
- Access to an online learning membership site with educational videos and other content like pain relief techniques, papers with therapeutic scripts, etc
This is an opportunity to learn a different way of helping people from doctors who target the 10-minute consultation with fast, effective formal hypnosis techniques and sleight-of-mouth. It is possible to make change happen in 10-minutes.
Note that attending this course will not make you a certified hypnotherapist, but confer you the skills you will learn to use personally and in the context of guided meditations and relaxations that are commonplace now.
And what evidence do I have for stating that NLP is unproven?
Is there an up-to-date and sound systematic review of NLP?
The answer is yes.
This systematic review of NLP included 10 experimental studies. Five studies were RCTs and five were uncontrolled pre-post studies. Targeted health conditions were anxiety disorders, weight maintenance, morning sickness, substance misuse, and claustrophobia during MRI scanning. NLP interventions were mainly delivered across 4-20 sessions although three were single session. Eighteen outcomes were reported and the RCT sample sizes ranged from 22 to 106. Four RCTs reported no significant between group differences with the fifth finding in favour of NLP. Three RCTs and five pre-post studies reported within group improvements. Risk of bias across all studies was high or uncertain.
The authors concluded that there is little evidence that NLP interventions improve health-related outcomes. This conclusion reflects the limited quantity and quality of NLP research, rather than robust evidence of no effect. There is currently insufficient evidence to support the allocation of NHS resources to NLP activities outside of research purposes.
Surprised?
I am not!
I did not expect the COMAIH to allow critical thinking to get in the way of quackery-promotion.
Hurray, homeopaths have a new study to be jubilant about!
But how far can we trust its findings?
Let’s have a look.
The aim of this study was to evaluate the effects of homeopathy (H) as an adjunct to non-surgical periodontal therapy (NSPT) in individuals with type 2 diabetes (DMII) and chronic periodontitis (CP). Eighty individuals with CP and DM II participated in this randomized, double-blind, placebo-controlled study. They were randomly divided into two groups: control group (CG) and the test group (TG), and both groups received the NSPT. TG also received homeopathic therapy, including Berberis, Mercurius solubilis/Belladonna/Hepar sulphur and Pyrogenium, while CG received placebo, while the TG received placebos. Clinical and laboratorial examinations were evaluated at baseline and after 1, 6 and 12 months of treatment.
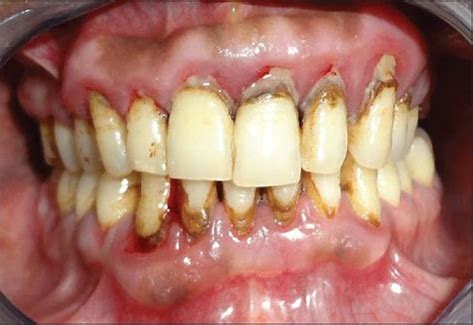
Both groups showed significant improvement throughout the study for most of the parameters studied, but TG presented a significative gain of clinical attachment at 1 and 12 months compared to CG. Mean glucose and glycated haemoglobin significantly decreased in both groups after 6 and 12 months. However, there was a significant further reduction of these parameters in TG, as compared to CG.
The authors concluded that homeopathy as supplement of NSPT may further improve health condition, including glycemic control, in DMII patients with CP.
Over the years, I have learnt how to ‘sniff out’ studies that are odd. This is one of them, I fear; it smells strangely ‘fishy’.
Here are some of the reasons why I remain sceptical:
- There does not seem to be an approval by an ethics committee.
- I also could also not find any mention of informed consent.
- There is no mention of conflicts of interest
- Neither is the source of funding disclosed.
- There were zero drop-outs which I find hard to believe.
- The trial started in 2013, but was published only recently.
- The treatment with homeopathy lacks biological plausibility.
- The authors conducted > 50 tests for statistical significance without correcting for multiple testing.
- The clinical relevance of the findings is unclear.
Even if we accepted the results of this study, we would require at least one independent replication before we allow them to influence our clinical practice.
I have been sceptical about Craniosacral Therapy (CST) several times (see for instance here, here and here). Now, a new paper might change all this:
The systematic review assessed the evidence of Craniosacral Therapy (CST) for the treatment of chronic pain. Randomized clinical trials (RCTs) assessing the effects of CST in chronic pain patients were eligible. Pain intensity and functional disability were the primary outcomes. Risk of bias was assessed using the Cochrane tool.

Ten RCTs with a total of 681 patients suffering from neck and back pain, migraine, headache, fibromyalgia, epicondylitis, and pelvic girdle pain were included.
Compared to treatment as usual, CST showed greater post intervention effects on:
- pain intensity (SMD=-0.32, 95%CI=[−0.61,-0.02])
- disability (SMD=-0.58, 95%CI=[−0.92,-0.24]).
Compared to manual/non-manual sham, CST showed greater post intervention effects on:
- pain intensity (SMD=-0.63, 95%CI=[−0.90,-0.37])
- disability (SMD=-0.54, 95%CI=[−0.81,-0.28]) ;
Compared to active manual treatments, CST showed greater post intervention effects on:
- pain intensity (SMD=-0.53, 95%CI=[−0.89,-0.16])
- disability (SMD=-0.58, 95%CI=[−0.95,-0.21]) .
At six months, CST showed greater effects on pain intensity (SMD=-0.59, 95%CI=[−0.99,-0.19]) and disability (SMD=-0.53, 95%CI=[−0.87,-0.19]) versus sham. Secondary outcomes were all significantly more improved in CST patients than in other groups, except for six-month mental quality of life versus sham. Sensitivity analyses revealed robust effects of CST against most risk of bias domains. Five of the 10 RCTs reported safety data. No serious adverse events occurred. Minor adverse events were equally distributed between the groups.
The authors concluded that, in patients with chronic pain, this meta-analysis suggests significant and robust effects of CST on pain and function lasting up to six months. More RCTs strictly following CONSORT are needed to further corroborate the effects and safety of CST on chronic pain.

Robust effects! This looks almost convincing, particularly to an uncritical proponent of so-called alternative medicine (SCAM). However, a bit of critical thinking quickly discloses numerous problems, not with this (technically well-made) review, but with the interpretation of its results and the conclusions. Let me mention a few that spring into my mind:
- The literature searches were concluded in August 2018; why publish the paper only in 2020? Meanwhile, there might have been further studies which would render the review outdated even on the day it was published. (I know that there are many reasons for such a delay, but a responsible journal editor must insist on an update of the searches before publication.)
- Comparisons to ‘treatment as usual’ do not control for the potentially important placebo effects of CST and thus tell us nothing about the effectiveness of CST per se.
- The same applies to comparisons to ‘active’ manual treatments and ‘non-manual’ sham (the purpose of a sham is to blind patients; a non-manual sham defies this purpose).
- This leaves us with exactly two trials employing a sham that might have been sufficiently credible to be able to fool patients into believing that they were receiving the verum.
- One of these trials (ref 44) is far too flimsy to be taken seriously: it was tiny (n=23), did not adequately blind patients, and failed to mention adverse effects (thus violating research ethics [I cannot take such trials seriously]).
- The other trial (ref 41) is by the same research group as the review, and the authors award themselves a higher quality score than any other of the primary studies (perhaps even correctly, because the other trials are even worse). Yet, their study has considerable weaknesses which they fail to discuss: it was small (n=54), there was no check to see whether patient-blinding was successful, and – as with all the CST studies – the therapist was, of course, no blind. The latter point is crucial, I think, because patients can easily be influenced by the therapists via verbal or non-verbal communication to report the findings favoured by the therapist. This means that the small effects seen in such studies are likely to be due to this residual bias and thus have nothing to do with the intervention per se.
- Despite the fact that the review findings depend critically on their own primary study, the authors of the review declared that they have no conflict of interest.
Considering all this plus the rather important fact that CST completely lacks biological plausibility, I do not think that the conclusions of the review are warranted. I much prefer the ones from my own systematic review of 2012. It included 6 RCTs (all of which were burdened with a high risk of bias) and concluded that the notion that CST is associated with more than non‐specific effects is not based on evidence from rigorous RCTs.
So, why do the review authors first go to the trouble of conducting a technically sound systematic review and meta-analysis and then fail utterly to interpret its findings critically? I might have an answer to this question. Back in 2016, I included the head of this research group, Gustav Dobos, into my ‘hall of fame’ because he is one of the many SCAM researchers who never seem to publish a negative result. This is what I then wrote about him:
Dobos seems to be an ‘all-rounder’ whose research tackles a wide range of alternative treatments. That is perhaps unremarkable – but what I do find remarkable is the impression that, whatever he researches, the results turn out to be pretty positive. This might imply one of two things, in my view:
- all alternative therapies are effective,
- the ‘Trustworthiness Index’ of Prof Dobos is unusual.
I let my readers chose which possibility they deem to be more likely.
As reported previously the NHS NATURAL HEALTH SCHOOL in Harrogate, is a service that offered a range of free complementary therapy treatments to patients and their relatives who are affected by a cancer diagnosis and are either receiving their cancer treatment at Harrogate or live in the Harrogate and Rural District.
This NHS school offered alternative treatments to cancer patients and claim that they know from experience, that when Complementary Therapies are integrated into patient care we are able to deliver safe, high quality care which fulfils the needs of even the most complex of patients.
In addition, they also ran courses for alternative practitioners. Their reflexology course, for instance, covered all of the following:
- Explore the history and origins of Reflexology
- Explore the use of various mediums used in treatment including waxes, balms, powders and oils
- Explore the philosophy of holism and its role within western bio medicine
- Reading the feet/hands and mapping the reflex points
- Relevant anatomy, physiology and pathology
- Managing a wide range of conditions
- Legal implications
- Cautions and contraindications
- Assessment and client care
- Practical reflexology skills and routines
- Treatment planning
I imagine that the initiators of the school are full of the very best, altruistic intentions. I therefore had considerable difficulties in criticising them. Yet, I do strongly feel that the NHS should be based on good evidence; and that much of the school’s offerings seemed to be the exact opposite. In fact, the NHS-label was being abused for giving undeserved credibility to outright quackery.
Therefore, I did something I do rarely: I filed an official complaint in September 2019.
What happened next?
Nothing!
I sent several reminders; and what happened then?
Almost nothing!
I got several assurances that a response was imminent.
And then I forgot all about it.
So, I was surprised to receive this email yesterday from the chief executive of the HARROGATE AND DISTRICT NHS FOUNDATION TRUST (I did not change or correct anything):
Thank you for contacting our Chair about the Natural Health School and my apologies for the extended delay in replying to you. We have reflected on the points you raised and I have set out a summary of this below in respect of the key issues.
- As a result of colleagues who set up the service having moved on to other posts outside of the Trust we have not been able to understand how the service was named. However, I agree that the terminology “NHS Natural Health School” could be interpreted in a certain way and as such we have agreed it should instead be referred to as the Natural Health School only to avoid any interpretation that it has national NHS endorsement. We will amend the information on the website and other material to reflect that the service is endorsed by the Trust.
- The service is hosted by HDFT, in that staff are employed by the Trust, but it is funded through charitable contributions. No NHS resources are used in providing the school, or the complementary therapies which are provided to patients receiving treatment at the Sir Robert Ogden Centre.
- There is no intention to assert that the services provided (ie the complementary therapies) are treatment for cancer. The ‘treatments’ referred to are complementary therapy treatments and are described as such. They are focused on wellbeing concurrently to the medical treatment of cancer, and we are satisfied that this is clear in the current description.
- Whilst recognising the differences of views on the complementary therapy treatments, the service regularly secures feedback from patients and this has been positive and as such we continue to offer it to those patients who would wish to take it up.
- The school provides training to allow participants to achieve a qualification which is awarded at level 3 by the International Therapies Examination Council.
I hope this provides clarity on the context to the service.
Best wishes
… … …
___________________________________________________________________
I find this response more than a little unsatisfactory; here are just a few points I find worth mentioning:
- As far as I can see, apart of the actual name of the school (it is now called ‘NATURAL HEALTH SCHOOL’), very little has changed. In particular, a NHS link is still implied in multiple different ways.
- To claim that ‘we have not been able to understand how the service was named’ seems like someone is taking the Mikey.
- So is the remark that ‘the terminology “NHS Natural Health School” could be interpreted in a certain way’.
- The statement ‘there is no intention to assert that the services provided (ie the complementary therapies) are treatment for cancer’ is simply untrue; symptomatic treatment of cancer is still a treatment for cancer!
- If the treatments are focussed on wellbeing, they nevertheless should be backed by evidence to show that they improve wellbeing. The label ‘complementary’ does not absolve a therapy from the need to be evidence-based.
- There may be ‘different views’ on complementary therapies; yet, there is only one set of evidence – and that fails to support several of the treatments on offer.
- Positive feedback from patients is no substitute for evidence.
I am not sure whether I should reply to the above letter. I take little pleasure in embarrassing chief excecutives.
WHAT DO YOU THINK I SHOULD DO?
Yesterday, we discussed a paper concluding (amongst other things) that there are insufficient high‐quality RCTs to judge the efficacy of acupuncture for cancer‐related pain. Today, we are looking at one that overtly contradicts this verdict.
This systematic review (published in JAMA Oncology) evaluated the existing randomized clinical trials (RCTs) for evidence of the association of acupuncture and acupressure with reduction in cancer pain. Randomized clinical trials that compared acupuncture and acupressure with a sham control, analgesic therapy, or usual care for managing cancer pain were included. The primary outcome was pain intensity measured by the Brief Pain Inventory, Numerical Rating Scale, Visual Analog Scale, or Verbal Rating Scale.
A total of 17 RCTs (with 1111 patients) were included, and data from 14 RCTs (with 920 patients) were used in the meta-analysis. Seven sham-controlled RCTs (35%) were notable for their high quality, being judged to have a low risk of bias for all of their domains, and showed that real (compared with sham) acupuncture was associated with reduced pain intensity. A favourable association was also seen when acupuncture and acupressure were combined with analgesic therapy in 6 RCTs for reducing pain intensity and in 2 RCTs for reducing opioid dose. The evidence grade was moderate because of the substantial heterogeneity among studies.
The authors concluded that this systematic review and meta-analysis found that acupuncture and/or acupressure was significantly associated with reduced cancer pain and decreased use of analgesics, although the evidence level was moderate. This finding suggests that more rigorous trials are needed to identify the association of acupuncture and acupressure with specific types of cancer pain and to integrate such evidence into clinical care to reduce opioid use.
So, which of the two conclusions should we trust?
Personally, I find the JAMA paper unimpressive to the point of being suspect. Here are some of my reasons:
- About half of the primary studies are Chinese; and we have seen repeatedly that they are unreliable and report only positive results.
- Many of the trials are published in Chinese and can thus not be checked by non-Chinese readers (nor, presumably, by the experts who acted as peer-reviewers for JAMA Oncology).
- I have my doubts about the rigor of the peer-review of some of the journals that published the primary studies included in the review.
- One paper included in the review is even a mere doctoral thesis which usually is not peer-reviewed in the usual sense.
- The authors state that they included only clinical trials that compared acupuncture and acupressure with a sham control, analgesic therapy, or usual care. However, this is evidently not true; many of the studies had the infamous A+B versus B design comparing acupuncture plus a conventional therapy against the conventional therapy. As we have discussed ad nauseam on this blog, such trials cannot produce a negative finding even if ‘A’ is a placebo.
- Contrary to what the authors claim, the quality of most of the included studies was extremely poor, as far as I can see.
- One included paper which I cannot access is entitled ‘Clinical observation on 30 cases of moderate and severe cancer pain of bone metastasis treated by auricular acupressure‘. Are the review authors seriously claiming that this is an RCT?
The more I study the details of the JAMA Oncology paper, the more I feel it might be worth a complaint to the editor with a view of initiating a thorough investigation and a possible retraction.
The aim of this review is to synthesise systematic reviews (SRs) of randomised clinical trials (RCTs) evaluating the efficacy of acupuncture to alleviate chronic pain. A total of 177 reviews of acupuncture from 1989 to 2019 met the eligibility criteria. The majority of SRs found that RCTs of acupuncture had methodological shortcomings, including inadequate statistical power with a high risk of bias. Heterogeneity between RCTs was such that meta-analysis was often inappropriate.
Having (co-) authored 13 of these SRs myself, I am impressed with the amount of work that went into this synthesis. The authors should be congratulated for doing it – and for doing it well! The paper itself differentiates the findings according to various types of pain. Here I reproduce the authors’ conclusion regarding different pain entities:
- Evidence from SRs suggests that there are insufficient high‐quality RCTs to judge the efficacy of acupuncture for chronic pain associated with various medical conditions. There is no specific NICE guidance about the use of acupuncture for chronic pain conditions irrespective of aetiology or pathophysiology, although some guidance exists for specific pain conditions (see respective sections below). Guidance by NICE on chronic pain assessment and management is currently being developed (GIDNG10069) with publication expected in August 2020.
- Evidence from the SRs suggests that acupuncture prevents episodic or chronic tension‐type headaches and episodic migraine, although long‐term studies and studies comparing acupuncture with other treatment options are still required. The current NICE guidance (clinical guideline CG150) is that a course of up to 10 sessions of acupuncture over 5–8 weeks is recommended for tension‐type headache and migraine.
- The most recent evidence from a Cochrane review of 16 RCTs suggests that acupuncture is not superior to sham acupuncture for OA of the hip, although in contrast, evidence from nonCochrane reviews suggests that there is moderate‐quality evidence that acupuncture may be effective in the symptomatic relief of pain from OA of the knee. Why there should be a difference in evidence between the knee and the hip is not known. Interestingly, guidance from NICE (CG177) states: “Do not offer acupuncture for the management of osteoarthritis”.
- Evidence suggests that there are insufficient high‐quality RCTs to judge the efficacy of acupuncture for low back pain. In 2009, NICE published guidance for the management of nonspecific low back pain that recommended a course of acupuncture as part of first line treatment. This guidance produced much debate. Subsequently, NICE have updated guidance for the management of low back pain and sciatica in people over 16 (NG59) and currently recommend in Section 1.2.8 “Do not offer acupuncture for managing low back pain with or without sciatica”, even though the evidence had not significantly changed.
- Evidence from SRs suggests that dry needling acupuncture might be effective in alleviating pain associated with myofascial trigger points, at least in the short‐term, although there are insufficient high‐quality RCTs to judge the efficacy with any degree of certainty. There is no guidance from NICE on the management of myofascial pain syndrome.
- Evidence from the SRs suggests that there are insufficient high‐quality RCTs to judge the efficacy of acupuncture for cancer‐related pain and more high‐quality, appropriately designed and adequately powered studies are needed. The most recent guidance from NICE (CSG4) recognises that patients who are receiving palliative care often seek complementary therapies, but it does not specifically recommend acupuncture. It recognises that “Many studies have a considerable number of methodological limitations, making it difficult to draw definitive conclusions”.
- Evidence from SRs suggests that there are insufficient high‐quality RCTs to judge the efficacy of acupuncture for fibromyalgia pain. There is no NICE guidance on the treatment of fibromyalgia.
- Evidence from the SRs suggests that there are insufficient high‐quality RCTs to judge the efficacy of acupuncture for primary dysmenorrhea or chronic pelvic pain. There is NICE guidance on endometriosis (NG73) [200] but this does not recommend any form of Chinese medicine for this type of pelvic pain, although acupuncture is not specifically mentioned.
- Evidence from the SRs suggests that there are insufficient high‐quality RCTs to judge the efficacy of acupuncture for pain in inflammatory arthritis. There is a NICE guideline (NG100) [201] for the treatment of rheumatoid arthritis but this does not recommend acupuncture.
- Evidence from the SRs suggests that there are insufficient high‐quality RCTs to judge the efficacy of acupuncture for neuropathic pain or neuralgia. There is NICE guidance (CG173) on the management of neuropathic pain, but acupuncture is not included in the list of recommended/not recommended treatments.
- Evidence from SRs suggests that there are insufficient high‐quality RCTs to judge the efficacy of acupuncture for a variety of other painful conditions, including lateral elbow pain, shoulder pain and labour pain. There is no guidance available from NICE on the treatment of any of these conditions.
So, what should we make of all this?
Maybe I just point out two things:
- This is a most valuable addition to the literature about acupuncture. It can serve as a reference for all who are interested in an honest account of the (lack of) value of acupuncture in the management of chronic pain.
- If a therapy has been tested in hundreds of (sadly often flawed) trials and the conclusions fail to come out clearly in favour of it, it is most likely not a very effective treatment.
Until we have data to the contrary, acupuncture should not be considered to be an effective therapy for chronic pain management.
If you, like many of us, have heavily ‘toxed’, you might now consider ‘detoxing’. What I mean is that we have probably all over-indulged a bit over the holidays. Unless you were the guest of someone, you had to pay dearly for it (Champagne is not cheap!). And now, a whole industry of ‘detox’ entrepreneurs tells you to pay again – this time, for detox.
As you payed ‘through your nose’ for the ‘tox’, you might as well use the same orifice for the ‘detox’. An Indian tradition called Nasiyam (or Nasyam? or Nasya? – I am confused!) makes it possible. This website explains:
Nasal Instillation (Nasyam) is the practice of instilling medicated oils, fresh juices of leaves or flowers in the nostrils … Nasyam is specially directed towards the purification of various parts related to the head…
I don’t know about you, but I always felt that all my parts were related to my head! So, Nasyam is for purification of all my parts? The announcement below – I picked it up on Twitter – is much clearer: detox through the nasal doorway! Who would refuse such an offer after the festivities of late?

This sounds fascinating, I thought. Thus I ran a quick Medline search but only found this abstract:
BACKGROUND:
Ardita (facial paralysis) is a medical condition that disfigures or distorts the facial appearance of the sufferer causing facial asymmetry and malfunction. Ardita patients may benefit from considering alternative treatments such as Ayurveda, including Taila Nasya (nasal instillation of medicated oil).
OBJECTIVES:
To synthesize the best available evidence on the effectiveness of different Nasya oils in the treatment of Ardita.
INCLUSION CRITERIA TYPES OF PARTICIPANTS:
Studies conducted on adult sufferers (18-70 years) of Ardita (chronic or acute) in any setting were considered. Studies including participants who were pregnant or suffered allergic rhinitis, fever, intracranial tumor/hemorrhage and bilateral facial palsy were excluded.
INTERVENTION(S)/COMPARATOR(S):
Standalone treatment of Nasya (at all dosages and frequencies) compared to Nasya in combination with other Ayurvedic treatments was considered. Comparisons between different interventions including Taila Nasya alone, Taila Nasya in combination with other Ayurvedic interventions and Ayurvedic interventions that did not include Taila Nasya were also considered.
OUTCOMES AND MEASURES:
Changes in Ardita symptoms, including facial distortion, speech disorders and facial pain, were measured.
TYPES OF STUDIES:
All quantitative study designs (experimental, quasi-experimental and observational) were considered.
SEARCH STRATEGY:
Relevant studies were identified following a comprehensive literature search. References provided within these key studies identified additional resources. Indian universities were also contacted for results of Ardita studies undertaken in their institutions.A three-step search strategy aimed to find studies of published and unpublished studies was undertaken. Studies published in the English language were considered for inclusion, irrespective of publication date/year. Following an initial limited search of MEDLINE and CINAHL, the text words contained in the title and abstract, and of the index terms used to describe each articles were analyzed. From the identified keywords and index terms, searches were undertaken across all relevant databases such as PubMed, CINHAL, Cochrane (CENTRAL), Scopus, Centre for Review and Dissemination databases, Turning Research into Practice (TRIP), EMBASE, EBM Reviews, DHARA, Google Scholar, MedNar and ProQuest Dissertations. Finally, reference lists of identified theses and articles were searched for additional studies. Universities and website operators related to Ayurvedic research in India were contacted, including the National Institute of Ayurveda for relevant studies. Besides this, the University of Adelaide librarian was contacted to retrieve those studies identified in the reference lists of theses and articles.
METHODOLOGICAL QUALITY:
Studies were critically assessed by the review author and a secondary reviewer prior to inclusion in the review using the standardized critical appraisal instrument from the Joanna Briggs Institute.
DATA EXTRACTION:
Data was extracted by the primary reviewer using the standardized data extraction tool from the Joanna Briggs Institute.
DATA SYNTHESIS:
Different interventions and comparators across studies precluded meta-analysis. Narrative synthesis was performed.
RESULTS:
Only two pseudo randomized studies with a small number of participants met inclusion criteria and were included in the review. One study with 20 participants, divided equally into two groups compared the effectiveness of two nasal instillations in alleviating four Ardita symptoms. The second study of 15 participants each in two groups compared the effectiveness of nasal instillation with placement of medicated oil on the head on seven Ardita symptoms. Observational measurements of Ardita symptoms were graded as Mild, Moderate or Marked at baseline and after one month. The study conducted on 30 participants using Nasya intervention showed participants had better relief from the symptoms of facial pain, speech disorder and earache within the range of 78.2% to 90.9%, graded as Marked. Along with statistical data available in the studies, this review found low levels of evidence favoring Taila Nasya intervention. The review did not include any studies examining effectiveness of Nasya compared to conventional treatment for Ardita.
CONCLUSIONS:
This review presents extremely limited evidence from only two small experimental studies that administration of Nasya oil alone may provide some relief from Ardita symptoms of facial distortion, speech disorder, inability to shut eyelids/upward eye rolling and dribbling of saliva in adult patients. No strong conclusions may be drawn from the evidence included in the review due to the limited number of studies, limited number of participants and poor quality of studies.
IMPLICATIONS FOR PRACTICE:
Practitioners should advice Ardita patients that there is extremely limited evidence suggesting the potential effectiveness of Nasya oils alone or Nasya in conjunction with other Ayurvedic treatments in managing symptoms. However, given the absence of a strong evidence base, practitioners should be guided by clinical wisdom and patient preference.
IMPLICATIONS FOR RESEARCH:
Well controlled clinical trials comparing standalone Nasya therapy to other Ayurvedic treatments and/or conventional medicine for Ardita symptoms need to be conducted to examine the relative effectiveness of different Nasya oils in treating.
I think you agree, that’s nothing to write home about.
So, on second thought I might give Nasya (or whatever it is called) a miss. The same applies, by the way, to any other form of detox.
In so-called alternative medicine (SCAM), there is never a dull moment; for me, the last decade was hardly an exception.
2010: Simon Singh and I had just published our book ‘Trick of Treatment‘(see also below), and consequently, the SCAM community (after having been more than a little suspicious of me for years) decided to oust me. At my university, I had major battles after the complaint by Prince Charles’ private secretary regarding the ‘Smallwood report‘. I decided to preserve my department by going into early retirement.
2011: This plan did not work out. I did retire, but the department sadly was closed down. The deal was that I get re-employed by my university on a half-time basis and help to find and appoint a successor.
2012: This did not work out either. We did find one suitable candidate, but they offered him terms that were totally unacceptable. The result: no more ‘Complementary Medicine’ at Exeter (I must admit, that was tough!). My wife and I thus sold our flat in Exeter and moved into rural Suffolk.
2013: My wife fell seriously ill, and we decided to move to France for an entire year to get her cured. This turned out to be the right decision; today she is alive and kicking.
2014: To prevent slowly going insane over all this, I had started writing my memoir.
2015: The book was published under the title ‘A scientist in wonderland’ and got unbelievably positive reviews. Today, this memoir is available also in German, Spanish and Korean. In the same year, I received the ‘John Maddox Award for standing up for science‘. I donated the prize money to the ‘Good Thinking Society’.
2016: While lecturing in Germany, I was invited by Springer to publish a book with them, and I chose the subject of homeopathy. The book entitled ‘Homeopathy, the undiluted facts‘ was later also published in German.
2017: I was awarded an Ockham Award at the QED. Sadly, I could not attend in person but Simon Singh received it in my name. We sold our house in Suffolk, moved to Cambridge, and also spent much of our time in France.
2018: After a perfect co-operation with the ethicist, Kevin Smith‘, our Springer book on the ethical issues raised by SCAM was published. It is entitled ‘More good than harm? The moral maze of complementary and alternative medicine‘ and received an award from the BMA. The same year, I also published my book ‘SCAM‘ which shortly after also was published in German.
2019: I published, again with Springer, an analysis of 150 SCAM modalities. Ioannidis et al published an analysis of 100 000 scientists of all fields. It implied that I am the most ‘influential’ researcher in the area of SCAM. This came just as unexpected as the US ‘Bookauthority’ naming our book ‘Trick or Treatment’ amongst the ‘best mental health books of all times’.

(Oh, I almost forgot: I also published ~500 Medline-listed papers as well as >1 700 blog posts and gave about 300 invited lectures during the last 10 years. Retirement turned out to be busy indeed.)
Sadly, the last decade also meant losing several good friends. John Dormandy and Gustav Born are two I miss very badly.
Yes, it was a decade full of action, worries, happiness, achievements and also true sadness. I hope I will still be around in 10 years to report about the next one.
I WISH ALL MY READERS A HAPPY AND HEALTHY NEW YEAR.
