This multi-center, open-label, randomized controlled trial assessed the effects of anthroposophic treatments on toxicity related to intensive-phase chemotherapy treatment in children aged 1-18 with the primary outcome of the toxicity sum score. Secondary outcomes were chemotherapy-related toxicity, overall and event-free survival after 5 years in study patients.
The main sponsorship for the study was provided by: Helixor Heilmittel GmbH & Co. KG, Rosenfeld. Additional finacial support was provided by: WALA Heilmittel GmbH, Bad Boll/Eckwälden; Weleda AG, Schwäbisch Gmünd: Mahle Stiftung, Stuttgart; Software AG Stiftung, Darmstadt; Stiftung Helixor, Rosenfeld; and Injex Pharma AG, Berlin.
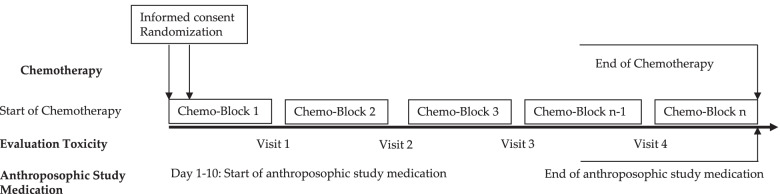
The intervention and control groups were both given standard chemotherapy according to malignancy & tumor type. The intervention arm was provided with anthroposophic supportive treatment (AST); given as anthroposophic base medication (AMP), as a base medication for all patients, and additional on-demand treatment tailored to the patient in the intervention groups. The control was given no AMP. The toxicity sum score (TSS) was assessed using NCI-CTC scales.
The AST consisted of base AMP including Helixor®, and on-demand supplementary AMP given as needed for symptoms. Administration of the AST intervention and chemotherapy protocol were tailored for each type of pediatric malignancy included in the trial. This included both the base and the on-demand AMP, which were administered based on acute symptoms during intensive chemotherapy. The intervention group started the AST between the day of randomization and day 10 of the first chemotherapy cycle.
Data of 288 patients could be analyzed. The analysis did not reveal any statistically significant differences between the AST and the control group for the primary endpoint or the toxicity measures (secondary endpoints). Furthermore, groups did not differ significantly in the five-year overall and event-free survival follow-up.
The authors concluded that their findings showed that AST was able to be safely administered in a clinical setting, although no beneficial effects of AST between group toxicity scores, overall or event-free survival were shown.
In their discussion section, the authors explain the findings more clearly: “In the long term follow up, the explorative analysis of the data available for the 5-year follow up found no indications that efficacy of chemotherapy was influenced by AST. For long-term toxicities there were also no indications of an influence of AST.”
Question: what do we call a treatment that has neither adverse nor beneficial effects?
Could it be
PLACEBO?

Question: what do we call a treatment that has neither adverse nor beneficial effects?
Could it be
PLACEBO? Question: what do we call a treatment that has neither adverse nor beneficial effects?
Could it be
PLACEBO?
I would prefer the term
USELESS