On Twitter, the hype had begun even before its text was available. Priti Gandhi, for instance, tweeted:
Yet another feather in India’s cap!! 1st evidence-based, CoPP-WHO GMP certified medicine for Covid-19 released today. Congratulations to @yogrishiramdev ji, @Ach_Balkrishna ji & the team of scientists at Patanjali Research Institute. Your efforts have been successful!! #Ayurveda
So, what is it all about? This study included 100 patients and was designed to evaluate the impact of traditional Indian Ayurvedic treatment on asymptomatic patients with COVID-19 infection. It is a placebo-controlled randomized double-blind pilot clinical trial that was conducted at the Department of Medicine in the National Institute of Medical Sciences and Research, Jaipur, India.
- 1 g of Giloy Ghanvati (Tinospora cordifolia)
- 2 g of Swasari Ras (traditional herbo-mineral formulation)
- 0.5 g of Ashwagandha (Withania somnifera)
- 0.5 g of Tulsi Ghanvati (Ocimum sanctum)
The treatment was given orally to the patients in the treatment group twice per day for 7 days. Medicines were given in the form of tablets and each tablet weighed 500 mg. While Swasari Ras was administered in powdered form, 30 min before breakfasts and dinners, rest were scheduled for 30 min post-meals. Patients in the treatment group also received 4 drops of Anu taila (traditional nasal drop) in each nostril every day 1 h before breakfast. Patients in the placebo group received identical-looking tablets and drops, post-randomization, and double-blinded assortments. 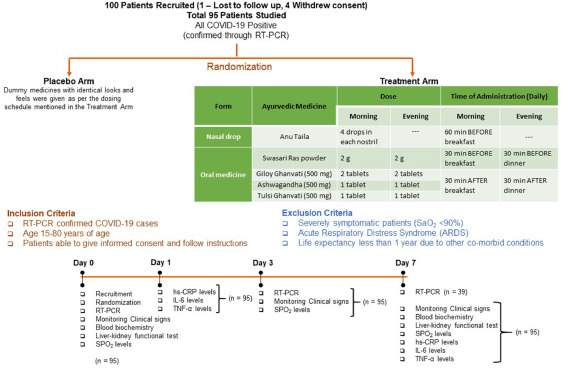 The RT-qPCR test was used for the detection of viral load in the nasopharyngeal and oropharyngeal swab samples of study participants during the study. Chemiluminescent immunometric assay was used to quantify serum levels of interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), and high sensitivity C-reactive protein (hs-CRP) on day 1 and day 7 of the study. Patient testing negative for SARS-CoV-2 in the RT-PCR analysis was the primary outcome of this study.
The RT-qPCR test was used for the detection of viral load in the nasopharyngeal and oropharyngeal swab samples of study participants during the study. Chemiluminescent immunometric assay was used to quantify serum levels of interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), and high sensitivity C-reactive protein (hs-CRP) on day 1 and day 7 of the study. Patient testing negative for SARS-CoV-2 in the RT-PCR analysis was the primary outcome of this study.
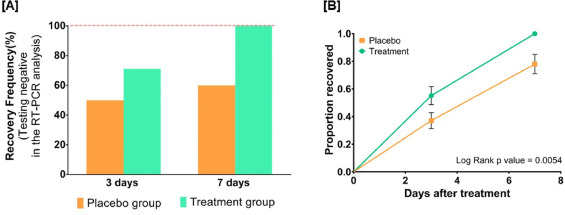
By day three, 71.1 % and 50.0 % of patients recovered in the treatment and placebo groups, respectively. The treatment group witnessed 100 % recovery by day 7, while it was 60.0 % in the placebo group. Average fold changes in serum levels of hs-CRP, IL-6, and TNF-α in the treatment group were respectively, 12.4, 2.5 and 20 times lesser than those in the placebo group at day 7. There was a 40 % absolute reduction in the risk of delayed recovery from infection in the treatment group.
The authors concluded that Ayurvedic treatment can expedite virological clearance, help in faster recovery and concomitantly reduce the risk of viral dissemination. Reduced inflammation markers suggested less severity of SARS-CoV-2 infection in the treatment group. Moreover, there was no adverse effect observed to be associated with this treatment.
I have the following concerns or questions about this trial:
- Why do the authors call it a pilot study? A pilot study is merely for testing the feasibility of a trial design and is not meant to yield definitive efficacy results.
- The authors state that the patients were asymptomatic yet in the discussion they claim they were asymptomatic or mildly symptomatic.
- Some of the effect sizes reported here are extraordinary and seem almost too good to be true.
- The claim of no adverse effect is implausible; even placebos would cause perceived adverse effects in a percentage of patients.
- If the study is solid and withstands the scrutiny of the raw data, it is of huge relevance for public health. So, why did the authors publish it in PHYTOMEDICINE, a relatively minor and little-known journal?
An article in The Economic Times’ reported this:
Patanjali Ayurved released what it called the first “evidence-based” medicine for Covid-19 on Friday. It claimed it has been “recognised by the WHO (World Health Organization) as an ayurvedic medicine for corona”.
Patanjali promoter, yoga guru Baba Ramdev, released a scientific research paper in this regard at the launch, presided over by Union health minister Harsh Vardhan and transport minister Nitin Gadkari.
The Ayurveda products maker said it has received a certification from the Ayush ministry. “Coronil has received the Certificate of Pharmaceutical Product (CoPP) from the Ayush section of Central Drugs Standard Control Organisation (CDSCO) as per the WHO certification scheme,” it said in a statement.
Under the CoPP, Coronil can be exported to 158 countries, the company said, adding that based on the presented data, the ministry has recognised Coronil as medicine for “supporting measure in Covid-19”.
Am I the only one who fears that something is not entirely kosher about the study? (This is an honest question, and I would be pleased to receive answers from my readers)

You are absolutely right to ask these questions. This paper is replete with flaws.
1. The reference in the Introduction where the claim is made that “…Ayurvedic medicines were effective against 2006 outbreak of Chikungunya Epidemic in India (Girija and Sivan, 2020).” is clearly an error That Girija and Sivan 2020 paper is in fact a case report of a New York Banker who recovered from his Covid-19 illness while taking Ayurvedic medication prescribed remotely from Chennai, India
2. Having presented the time to viral clearance as Kaplan Meier curves, the authors then artificially construct a binary outcome of event rates where the event is defined as virus cleared at 7 days. No confidence rates for the relative rate reductuiion or NNTs.
3. Lead time bias. Since the patients enrolled are largely asymptomatic, to count the time to clearance from the day of testing is to ignore how long before the day of testing the patient might have had the virus for. If this lead time differed systematically between the 2 groups then the conslusion would be biased.
Few things are immediately apparent.
If we look at figure 6A, for example, we discover that for the placebo group at day 7 there are 48 dots reported, but from table 1 we learn that in the placebo group there were 50 people enrolled.
Similarly, for the treated group on day 7, you have values for 43 individuals, while 45 people were treated.
As for figure 3B, we see that error bars are reported for the proportion of patients who recovered: what those error bars stand for?
In table 1, you have p values which should be related to the differences among the average values of several parameters among the placebo and the control groups. However, in the table, you find things like Respiratory Rate equal to 17 (12, 20) for the placebo group, and 17 (12, 20) for the treated group (so identical average values and max/min for the distributions). Besides the fact that such a degree of similarity among two groups of about 50 people is unrealistic, how can you have p=0.41 (as if there was some degree of difference between the two groups)?
There are several more discrepancies, but I think this is enough to give you a feeling for it.
several things
I cannot access details of the full study just the abstract so have no idea what kind of shenanigans might have gone on with the trial protocol or the blinding or the randomozation or the statistical analysis. For all we know they might have done it all on the back of a fag packet.
100 patients is not a big study to be making a big hoopla about. Do it again with more patients.
You shouldn’t go off half-cocked and be issuing WHO treatment certificates for Covid-19 “cures” on the basis of a half-baked study liked this. What happened to replication?
The confusion about “pilot study” and asymptomatic and symptomatic” patients just lends credence to the idea that these AYUSH guys don’t really have a clue what they are doing – the vast majority of alt-med research is badly done and is performed not with the aim of seeking truth but with the aim of proving their SCAM “works.”
Any drug with 100% efficacy and 0% side effects sounds more like a miracle than a treatment. If it sounds too good to be true……..As Edzard says even placebos have side-effects.
Prior plausibility – there is no reason to imagine that Anu taila should have miraculous properties for fending off Covid-19
so this reduces PP to 0. Ayurveda is pre-scientific fantasy not real world medicine.
“Under the CoPP, Coronil can be exported to 158 countries, the company said, adding that based on the presented data, the ministry has recognised Coronil as medicine for “supporting measure in Covid-19”.
The rush to get the above out heavily endorsed by ministers sounds like drug company blurb and is science free promotional guff – which probably makes it not very different from the “study.”
If it had any real basis in fact the NEJM would have bitten their hand off.
The ingredients used in this treatment:
– Ghanvati (Tinospora cordifolia): “Despite centuries of use in traditional medicine to treat various disorders, there is no clinical evidence that it has an effect on any diseases”.
– Swasari Ras, consisting of
* Mulethi (licorice root): “there isn’t enough high-quality evidence to clearly support its use for any health condition”.
* Lavang (cloves): “The effectiveness ratings for CLOVE are as follows: Insufficient evidence to rate effectiveness for [all conditions listed]”.
* Dalchini (cinnamon): “Studies done in people don’t clearly support using cinnamon for any health condition”.
* Kakdasingi (Pistacia integerrima): no scientific data on medicinal efficacy available(*).
* Rudanti (Rudravanti): no scientific data on medicinal efficacy available.
* Sounth (Ginger): ginger may be helpful in cases of mild nausea, but has no known effect on any other condition.
* ChhotiPipal (Piper retrofractum): no scientific data on medicinal efficacy available.
* Abhrak Bhasma (mica ash): no scientific data on medicinal efficacy available.
* Mukta Shukti Bhasma (ground-up, calcined pearl oyster shell = calcium carbonate): somewhat effective as an antacid, no other indications.
* Kapardak Bhasma (ground-up, calcined cowrie shell = calcium carbonate): see above.
– Ashwagandha (Withania somnifera): “Although thought to be useful as a medicinal herb in Ayurveda and sold in many countries as a dietary supplement, there is insufficient scientific evidence that it is safe or effective for treating any disease”.
– Tulsi Ghanvati (Ocimum sanctum, ‘holy basil’): no scientific data on medicinal efficacy available.
*: This doesn’t stop alternative practitioners from making quite strong assertions such as this one: “They are of great medicinal value and they are used as a cure for diseases like: [grab-bag of totally unrelated conditions, including STD’s, asthma and epilepsy]”
Also note the wording further down: “… helps … is said to be … it is believed …”.
Any pharmaceutical company trying to sell their products with this type of ‘evidence’ of efficacy would be forced to close down immediately.
So basically what we have here are some common spices found in almost every kitchen cabinet, some more obscure herbs, and a dash of limestone powder. None of these ingredients has proven antiviral effects, and their efficacy as a Covid-19 medicine is highly dubious.
Small correction of the chemistry: calcined shells are not calcium carbonate (limestone) any more, but calcium oxide (lime).
So the mineral ingredients of this ayurvedic ‘medicine’ are in fact cement – yes, the same stuff that is used as a building material.
that’s what I call solid evidence!!!
Also, isn’t CaO + H2O -> Ca(OH)2 fairly exothermic? How much CaO is in a dose?
Yes, it is, but given that the dose of Swarasi Ras mentioned is a mere 2 grams, and quicklime makes up only part of it, I guess that the total dose of CaO is limited to some 100 mg maximum(*). The highly alkaline nature of the stuff is probably more of a problem for the upper digestive tract than the heat – although it is of course immediately neutralized upon entering the stomach. If taken in small enough doses with lots of water, it is probably not very harmful – which is more than can be said for other ayurvedic ‘medicines’ such as this highly toxic concoction.
*: The traditional small-scale calcining process is probably not all that efficient anyway, so there will be some amount of calcium carbonate left as well. Together with the minerals from the calcined mica, the resulting mineral mixture is virtually identical to cement.
I cured my eczema with Ayurveda and I have long covid and it’s working for this too, where the West just made me really ill, with meds and the way they spoke to me. I am a health professional and have done research previously and all research is different, there’s alot of sub standard western papers. It amazes me that the West a’d the East together could learn about this virus. The reason ayurveda and other alternatives have been given the go ahead for research is because everyone is lost. The NHS are trialling an ayurvedic herb to replace antibiotics, as they need help.
Previously other treatments were not granted funds, acknowledgement, hence the lack of research. I know, I’m in a health profession where the funding is low.
Why don’t we all work together and allow all therapies to give their point of view. It would be better for systems, patients and the world. That is if the answers/ cures is what people are looking for, if it is then there would be no judgement on others. Blessings
“Why don’t we all work together and allow all therapies to give their point of view?”
We do, except we insist that views are evidence-based; creeds and anecdotes are not helpful.
False claim in the comment related to Mulethi (licorice root).
Research published in the Lancet reported that glycyrrhizin, a major active constituent liquorice root which is the most frequently used Chinese herb, potently inhibited the replication of clinical isolates of SARS virus [Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. The Lancet. 2003;361:2045-6]
here’s another interesting finding about this paper. The authors claim that there was randomization and so the 2 groups would be largely similar in all respects. They are at pains to show this in Table 1 in the paper.
But they also make much of the finding that the IL-6 level showed a big change in the Treatment group. We don’t of course have the full data set but some limited raw data are presented in Table 4. This shows the IL-6 levels in pg/ml on Day1 and Day 7 in the two groups – Placebo and Treatment. I charted this data in R and ggplot to get: https://twitter.com/GorwayGlobal/status/1363124584078336000?s=20
Interesting. So the treatment were much more into their COVID journey than the placebo. As measured by IL 6 levels.
Perhaps we should also take a look on the promoting company of Coronil, Patanjali Ayurved.
“In June 2020, Patanjali Ayurved announced a drug named Coronil for COVID-19 treatment, Ramdev claimed in June that Coronil had cured Covid-19 patients. The Indian government has allowed Patanjali Ayurved to market Coronil as an immunity booster but not a cure. The Government of Maharashtra has banned the sale of Coronil in the state. Law suits were filed in Bihar and Rajasthan against Ramdev, Balkrishna, and others, accusing them of cheating and selling fake medicines. The Madras High Court has fined the company ₹1,000,000 (US$14,000) for its false claims on the drug. Patanjali has withdrawn the claim of Coronil being a cure for Covid-19. The UK drug regulator has threatened action if the unauthorized products were sold in the UK market.”
https://en.wikipedia.org/wiki/Patanjali_Ayurved#Fake_COVID-19_drug_development
The company does not make a very trustworthy impression.
It sounds like cargo cult science to me, taking a grab bag of assays, for no particular reason, and report the ones that change.
this post got me banned twice from Facebook;
I will now post it again to see whether I can manage a third time.
more about this trial here:
https://caravanmagazine.in/health/the-bad-science-and-poor-ethics-of-patanjali-coronil-research
There are thousands of patients who have cured their Covid-19 infections with this treatment plan. People who took this plan from Day 1 of their Covid-19 symptoms did not have to be hospitalized and recovered quickly. This is just the aversion of the West in acknowledging research based medicine coming out of the East. Quoting of inadequate references just to prove their point.
Giloy or Guduchi has been effective in stopping all kinds of infections. There is just so much research published on it. You can look up a few here – https://scholar.google.co.in/scholar?q=giloy+guduchi+benefits+research&hl=en&as_sdt=0&as_vis=1&oi=scholart#d=gs_qabs&u=%23p%3D5LJLRIUXv9EJ.
Ashwagandha benefits are just too many – Pretreatment with Withania somnifera (WS) showed significance protection against stress induced gastric ulcers. WS have anti-tumor effect on Chinese Hamster Ovary (CHO) cell carcinoma. It was also found effective against urethane induced lung-adenoma in mice. In some cases of uterine fibroids, dermatosarcoma, long term treatment with WS controlled the condition. It has a Cognition Promoting Effect and was useful in children with memory deficit and in old age people loss of memory. It was also found useful in neurodegenerative diseases such as Parkinson’s, Huntington’s and Alzeimer’s diseases. It has GABA mimetic effect and was shown to promote formation of dendrites. It has anxiolytic effect and improves energy levels and mitochondrial health. It is an anti-inflammatory and anti-arthritic agent and was found useful in clinical cases of Rheumatoid and Osteoarthritis. Large scale studies are needed to prove its clinical efficacy in stress related disorders, neuronal disorders and cancers. Reference – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3252722/
Swashari ras has been effective against all forms of Respiratory problems. It has been used in traditional medicine for centuries. Reference- https://www.sciencedirect.com/science/article/pii/S0753332220302547
Another covid-19 treatment that has not received much focus. This study was first completed in March of 2021, but not reviewed till more recently.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7987002/
https://www.science.org/doi/10.1126/sciadv.abi6110