This systematic review included 18 studies assessing homeopathy in depression. Two double-blind placebo-controlled trials of homeopathic medicinal products (HMPs) for depression were assessed. The first trial (N = 91) with high risk of bias found HMPs were non-inferior to fluoxetine at 4 (p = 0.654) and 8 weeks (p = 0.965); whereas the second trial (N = 133), with low risk of bias, found HMPs was comparable to fluoxetine (p = 0.082) and superior to placebo (p < 0.005) at 6 weeks.
The remaining research had unclear/high risk of bias. A non-placebo-controlled RCT found standardised treatment by homeopaths comparable to fluvoxamine; a cohort study of patients receiving treatment provided by GPs practising homeopathy reported significantly lower consumption of psychotropic drugs and improved depression; and patient-reported outcomes showed at least moderate improvement in 10 of 12 uncontrolled studies. Fourteen trials provided safety data. All adverse events were mild or moderate, and transient. No evidence suggested treatment was unsafe.
The authors concluded that limited evidence from two placebo-controlled double-blinded trials suggests HMPs might be comparable to antidepressants and superior to placebo in depression, and patients treated by homeopaths report improvement in depression. Overall, the evidence gives a potentially promising risk benefit ratio. There is a need for additional high quality studies.
It is worth having a look at these two studies, I think.
The 1st (2011) study is from Brazil
Here is its abstract:
Homeopathy is a complementary and integrative medicine used in depression, The aim of this study is to investigate the non-inferiority and tolerability of individualized homeopathic medicines [Quinquagintamillesmial (Q-potencies)] in acute depression, using fluoxetine as active control. Ninety-one outpatients with moderate to severe depression were assigned to receive an individualized homeopathic medicine or fluoxetine 20 mg day−1 (up to 40 mg day−1) in a prospective, randomized, double-blind double-dummy 8-week, single-center trial. Primary efficacy measure was the analysis of the mean change in the Montgomery & Åsberg Depression Rating Scale (MADRS) depression scores, using a non-inferiority test with margin of 1.45. Secondary efficacy outcomes were response and remission rates. Tolerability was assessed with the side effect rating scale of the Scandinavian Society of Psychopharmacology. Mean MADRS scores differences were not significant at the 4th (P = .654) and 8th weeks (P = .965) of treatment. Non-inferiority of homeopathy was indicated because the upper limit of the confidence interval (CI) for mean difference in MADRS change was less than the non-inferiority margin: mean differences (homeopathy-fluoxetine) were −3.04 (95% CI −6.95, 0.86) and −2.4 (95% CI −6.05, 0.77) at 4th and 8th week, respectively. There were no significant differences between the percentages of response or remission rates in both groups. Tolerability: there were no significant differences between the side effects rates, although a higher percentage of patients treated with fluoxetine reported troublesome side effects and there was a trend toward greater treatment interruption for adverse effects in the fluoxetine group. This study illustrates the feasibility of randomized controlled double-blind trials of homeopathy in depression and indicates the non-inferiority of individualized homeopathic Q-potencies as compared to fluoxetine in acute treatment of outpatients with moderate to severe depression.
There are many important points to make about this trial:
- Contrary to what the reviewers claim, the trial had no placebo group.
- It was a double-dummy equivalence study comparing individualised homeopathy with the antidepressant fluoxetine.
- Fluoxetine might have been under-dosed (see below).
- Equivalence studies require large sample sizes, and with just 91 patients (only 55 of whom finished the study), this trial was underpowered which means the finding of equivalence is false positive.
- The authors noted that a higher percentage of troublesome adverse effects reported by patients receiving fluoxetine. This means that the trial was not double-blind; patients were able to tell by their side-effects which group they were in.
- The authors also state that more patients randomized to homeopathy than to fluoxetine were excluded due to worsening of their depressive symptoms. I think this confirms that homeopathy was ineffective.
The 2nd (2015) study is from Mexico
Here is its abstract:
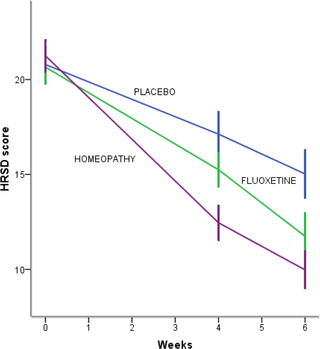
Background: Perimenopausal period refers to the interval when women’s menstrual cycles become irregular and is characterized by an increased risk of depression. Use of homeopathy to treat depression is widespread but there is a lack of clinical trials about its efficacy in depression in peri- and postmenopausal women. The aim of this study was to assess efficacy and safety of individualized homeopathic treatment versus placebo and fluoxetine versus placebo in peri- and postmenopausal women with moderate to severe depression.
Methods/Design: A randomized, placebo-controlled, double-blind, double-dummy, superiority, three-arm trial with a 6 week follow-up study was conducted. The study was performed in a public research hospital in Mexico City in the outpatient service of homeopathy. One hundred thirty-three peri- and postmenopausal women diagnosed with major depression according to DSM-IV (moderate to severe intensity) were included. The outcomes were: change in the mean total score among groups on the 17-item Hamilton Rating Scale for Depression, Beck Depression Inventory and Greene Scale, after 6 weeks of treatment, response and remission rates, and safety. Efficacy data were analyzed in the intention-to-treat population (ANOVA with Bonferroni post-hoc test).
Results: After a 6-week treatment, homeopathic group was more effective than placebo by 5 points in Hamilton Scale. Response rate was 54.5% and remission rate, 15.9%. There was a significant difference among groups in response rate definition only, but not in remission rate. Fluoxetine-placebo difference was 3.2 points. No differences were observed among groups in the Beck Depression Inventory. Homeopathic group was superior to placebo in Greene Climacteric Scale (8.6 points). Fluoxetine was not different from placebo in Greene Climacteric Scale.
Conclusion: Homeopathy and fluoxetine are effective and safe antidepressants for climacteric women. Homeopathy and fluoxetine were significantly different from placebo in response definition only. Homeopathy, but not fluoxetine, improves menopausal symptoms scored by Greene Climacteric Scale.
And here are my critical remarks about this trial:
- The aim of a small study like this cannot be to assess or draw conclusions about the safety of the interventions used; for this purpose, we need sample sizes that are at least one dimension bigger.
- Fluoxetine might have been under-dosed (see below).
- The blinding of patients might have been jeopardized by patients experiencing the specific side-effects of fluoxetine. The authors reported adverse effects in all three groups. However, the characteristic and most common side-effects of fluoxetine (such as hives, itching, skin rash, restlessness, inability to sit still) were not included.
________________________________________________
Usual Adult Dose for Depression
Immediate-release oral formulations:
Initial dose: 20 mg orally once a day in the morning, increased after several weeks if sufficient clinical improvement is not observed
Maintenance dose: 20 to 60 mg orally per day
Maximum dose: 80 mg orally per day
Delayed release oral capsules:
Initial dose: 90 mg orally once a week, commenced 7 days after the last daily dose of immediate-release fluoxetine 20 mg formulations.
_________________________________________________
Considering all this, I feel that the conclusions of the above review are far too optimistic and not justified. In fact, I find them misleading, dangerous, unethical and depressing.

” I find them misleading, dangerous, unethical and depressing.”
And entirely typical of the genre.
It really is time we moved on.
Perhaps we have?
The first study was published in 2011 and the second in 2015.
I charge that journal editors are to blame for lax, misleading, even fraudulent standards.
Edzard,
While I agree with your conclusions that the trials that you have detailed do not support the hypothesis that homeopathic treatment is effective for depression, or that it is equivalent to the SSRI’s used as controls, I am not sure that I agree with all of the points that you have made.
A great many clinical trials do not have a placebo group, but instead compare the new treatment with the current standard of care. Depending on the circumstances it might be unethical to deny the control group an established treatment that is known to work. Certainly it makes recruitment at lot easier if the subjects know that they will all be receiving treatment. For instance, supposing that you have a new hormonal drug and you want to test its efficacy in preventing the recurrence of breast cancer, then it would be normal to test it against an existing drug such as tamoxifen, which is known to be effective.
As you correctly point out, the lack of a statistically significant difference between the outcomes is not proof of non-inferiority. A non-inferiority trial needs to be set up from the beginning as such, which includes deciding in advance what the threshold for non-inferiority should be and calculating the sample size that will show this with a given level of significance; generally large numbers are required for this sort of trial. In the trials you have detailed, the only thing that the claims of non-inferiority idemonstrate is that the authors don’t understand the statistical tests that they are using.
To say that the trial is not blinded because the subjects can tell which group they are in from the side-effects they report could probably be applied to almost any trial of an effective treatment. We know that drugs and other interventions powerful enough to have desired effects also have undesirable effects, and indeed this is one of the things that all good clinical trials record. It is rather unusual for toxicities to be the same in treatment and control groups. However, generally the subjects are not sufficiently well-acquainted with the drugs in question to know what to look for, and there is also the nocebo effect to consider. Even if the person assessing the toxicity in a given patient forms their own view of which arm they are in, really it is only apparent at the time of analysis what is going on. The dermatological symptoms that you mention (hives – i.e. urticaria, itching and rashes) are very common, and restlessness and inability to sit still are frequent features of agitated depression, and I don’t think that in themselves they would be a giveaway in an individual patient.
I wonder if you could clarify something? In the first study, when the authors state that more patients randomised to homeopathy were excluded due to worsening depression, does that mean that they were not included in the analysis? If so, that makes a nonsense of the whole trial, as the analysis should have been on an intent-to-treat basis (i.e. which group they were randomised to) not on the basis of what treatment they actually received (which would introduce bias).
I completely agree with Richard Rawlins that these failings should be picked up by the journal editors and the peer review process before the trials are ever published.
“A great many clinical trials do not have a placebo group, but instead compare the new treatment with the current standard of care.”
I know that of course, yet I object to the review-authors calling it placebo-controlled.
“To say that the trial is not blinded because the subjects can tell which group they are in from the side-effects they report could probably be applied to almost any trial of an effective treatment.”
that’s why the success of blinding ought to be checked in such trials.
“I wonder if you could clarify something? In the first study, when the authors state that more patients randomised to homeopathy were excluded due to worsening depression, does that mean that they were not included in the analysis? ”
this is not entirely clear from the paper.
Seeing this exchange is heartening. Two professionals who know their subjects and hone Words for clarity provides far more access for this layman to understand. Thank you both.
One professional “challenged” by a charlatan generates a confused exchange where the latter relies on befuddlement to sneak their assertion through.