pseudo-science
Almost 10 years ago, I posted this:
When I decided to become a doctor I, like most medical students, did so mainly to help suffering individuals. When I became a researcher, I felt more removed from this original ideal. Yet I told myself that, by conducting research, I might eventually contribute to a better health care of tomorrow. Helping suffering patients was still firmly on the agenda. But then I realised that my articles in peer-reviewed medical journals somehow missed an important target: in alternative medicine, one ought to speak not just to health care professionals but also to consumers and patients; after all, it is they who often make the therapeutic decisions in this area.
Once I had realised this, I started addressing the general public by writing for The Guardian and other newspapers, giving public lectures and publishing books for a lay audience, like TRICK OR TREATMENT…The more I did this sort of thing, the more I noticed how important this activity was. And when a friend offered to help me set up a blog, I did not hesitate for long.
So, the reason for my enthusiasm for this blog turns out to be the same as the one that enticed me to go into medicine in the first place. I do believe that it is helpful for consumers to know the truth about alternative medicine. Considering the thousands of sources of daily misinformation in this area, there is an urgent need for well-informed, critical information. By providing it, I am sure I can assist people to make better therapeutic decisions. In a way, I am back where I started all those years ago: hoping to help suffering patients in the most direct way my expertise allows.
Helping vulnerable patients often means warning them from dangerous charlatans, and this is precisely what I frequently try to do with this blog. But how successful are my endeavors?
More often than not, I have no idea and can only hope for the best. Sometimes I do get some feedback that is encouraging and motivates me to carry on. Rarely, however, do I witness immediate, tangible success. And this is why the recent story is so remarkable:
- On 6 June, an Australian acquaintance from the FRIENDS OF SCIENCE IN MEDICINE sent me some material about a planned lecture in the UK by someone promoting dangerous quackery.
- I looked into it and published a blog post about it a few hours later.
- A reader then suggested in the comments section of this post alerting the UK press to it.
- Another reader contacted THE TIMES, and I wrote to several other journalists.
- THE TIMES turned out to be interested in the story.
- They did some research and interviewed Michael Marshall from the GOOD THINKING SOCIETY (and myself).
- Today, THE TIMES published an article about the planned event.
- Finally, a kind person made the article available to those who don’t want to pay for it.
The whole thing amounts to superb teamwork, in my view. It shows how like-minded people who do not even all know each other can manage to achieve a respectable result with little more than goodwill and dedication.
A respectable result?
Of course, the optimal result would be to stop Barbara O’Neill’s UK lectures. Let’s hope this is what eventually will happen – and please let me know if you know more.
This article almost left me speechless:
The back-to-back waves of the COVID-19 pandemic have made a devastating impact globally. The conventional healthcare system is going through serious pressure as cases of the disease continue to spread and the numbers of hospitalizations are increasing every moment. It is becoming hard and challenging because the hospital resources are limited in number as compared with the rate of daily hospitalizations. There are significant shortages of patient care facilities and medical care providers, and on top of that, conventional healthcare systems do not have any proven treatments for COVID-19 patients. Experimental drugs like hydroxychloroquine, followed by remdesivir, ritonavir/lopinavir, and favipiravir are being administered under emergency use authorization (EUA). There is evidence that these experimental medications are causing adverse drug reactions, thus claiming the lives of the hospitalized COVID-19 patients. And those patients who survive the EUA medications and hospitalizations are left with iatrogenic immunosuppressive states leading to increased susceptibility towards secondary life-threatening infections like fungal diseases. In this scenario, complementary and alternative medical systems (CAMS) are providing commendable results with negligible adverse effects or iatrogenic issues in patients with COVID-19. There are several clinical cases recorded and published by various independent homoeopathic doctors and researchers worldwide. But unfortunately, because of a biased medical model and greed for monopolies, these effective treatment methods are not given equal opportunity as their conventional counterparts.
I think the best way to react to this nonsense might be to remind us what the only RCT of homeopathy for COVID showed.
This randomized, double-blind, two-armed, parallel, single-center, placebo-controlled study investigated the effectiveness and safety of the homeopathic medicine, Natrum muriaticum LM2, for mild cases of COVID-19.
Participants aged > 18 years, with influenza-like symptoms and a positive COVID test were recruited and randomized (1:1) into two groups that received different treatments during a period of at-home isolation. One group received the homeopathic medicine Natrum muriaticum, prepared with the second degree of the fifty-millesimal dynamization (LM2; Natrum muriaticum LM2), while the other group received a placebo.
The primary endpoint was time until recovery from COVID-19 influenza-like symptoms. Secondary measures included a survival analysis of the number and severity of COVID-19 symptoms (influenza-like symptoms plus anosmia and ageusia) from a symptom grading scale that was informed by the participant, hospital admissions, and adverse events. Kaplan-Meier curves were used to estimate time-to-event (survival) measures.
Data from 86 participants were analyzed (homeopathy, n = 42; placebo, n = 44). There was no difference in time to recovery between the two groups (homeopathy, n = 41; placebo, n = 41; P = 0.56), nor in a sub-group that had at least 5 moderate to severe influenza-like symptoms at the beginning of monitoring (homeopathy, n = 15; placebo, n = 17; P = 0.06). Secondary outcomes indicated that a 50% reduction in symptom score was achieved significantly earlier in the homeopathy group (homeopathy, n = 24; placebo, n = 25; P = 0.04), among the participants with a basal symptom score ≥ 5. Moreover, values of restricted mean survival time indicated that patients receiving homeopathy might have improved 0.9 days faster during the first five days of follow-up (P = 0.022). Hospitalization rates were 2.4% in the homeopathy group and 6.8% in the placebo group (P = 0.62). Participants reported 3 adverse events in the homeopathy group and 6 in the placebo group.
The authors concluded that the results showed that Natrum muriaticum LM2 was safe to use for COVID-19, but there was no statistically significant difference in the primary endpoints of Natrum muriaticum LM2 and placebo for mild COVID-19 cases.
Another relevant study compared the antibody response of homeopathic and conventional vaccines and placebo in young adults. A placebo-controlled, double-blind RCT was conducted where 150 university students who had received childhood vaccinations were assigned to diphtheria, pertussis, tetanus, mumps, measles homeopathic vaccine, placebo, or conventional diphtheria, pertussis, tetanus (Tdap) and mumps, measles, rubella (MMR) vaccines. The primary outcome was a ≥ two-fold increase in antibodies from baseline following vaccination as measured by ELISA. Participants, investigators, study coordinators, data blood drawers, laboratory technicians, and data analysts were all blinded.
None of the participants in either the homeopathic vaccine or the placebo group showed a ≥ two-fold response to any of the antigens. In contrast, of those vaccinated with Tdap, 68% (33/48) had a ≥ two-fold response to diphtheria, 83% (40/48) to pertussis toxoid, 88% (42/48) to tetanus, and 35% (17/48) of those vaccinated with MMR had a response to measles or mumps antigens (p < 0.001 for each comparison of conventional vaccine to homeopathic vaccine or to placebo). There was a significant increase in geometric mean titres of antibody from baseline for conventional vaccine antigens (p < 0.001 for each), but none for the response to homeopathic antigens or placebo.
The authors concluded that homeopathic vaccines do not evoke antibody responses and produce a response that is similar to placebo. In contrast, conventional vaccines provide a robust antibody response in the majority of those vaccinated.
To give ‘equal opportunity’ to implausible therapies would, in my view, not merely be wrong, it would be scandalously unethical. The role of homeopathy in the prophylaxis and symptomatic management of COVID-19 or other infections is very easily described; it is:
zero,
nil,
nothing,
null,
naught,
zilch.
Bee venom acupuncture (BVA) is a bizarre form of acupuncture where bee venom is applied via a bee sting or an injection into acupuncture points. The paper below starts with the sentence: “BVA is an effective treatment method for various diseases.” This clearly is not true. In fact, there is no convincing evidence that it is effective for any condition. In addition, it can cause serious harm, even life-threatening anaphylaxis.
With this review, Korean authors tried to estimate the incidence rate of anaphylaxis in response to BVA. 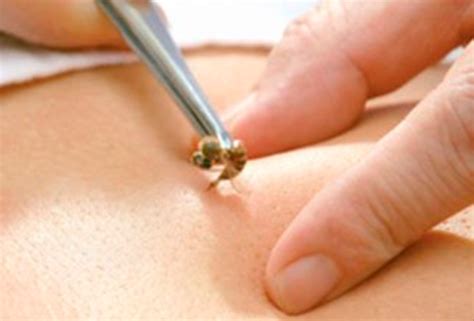
The investigators searched eight databases (MEDLINE (Pubmed), EMBASE, Cochrane Central Register of Controlled, KISS, KMBASE, Koreamed, OASIS, and NDSL) and systematically reviewed the articles that met the inclusion/exclusion criteria.
Among 225 potentially relevant articles, 49 were selected for this study. The overall incidence rate of anaphylaxis in response to BVA was 0.045% (95% CI 0.028-0.062). Women (0.083%, 95% CI 0.010-0.157) showed a higher incidence rate than men (0.019%, 95% CI -0.018 to 0.055), while the incidence for patients who had a skin test conducted (0.041%, 95% CI 0.011-0.072) was not significantly different compared to that obtained for patients for which there was no information about a skin test (0.047%, 95% CI 0.026-0.067). The publication year affected the incidence rate: it was highest before 1999 (1.099%, 95% CI -1.043 to 3.241), lower between 2000 and 2009 (0.049%, 95% CI 0.025-0.073), and lowest between 2010 and 2021 (0.037% 95% CI 0.014-0.060).
The authors concluded that, in this study, we provide reference data about risk size and factors of BVA-related anaphylaxis, which is essentially required for BVA application in clinics.
I fail to understand why this review included only observational studies and RCTs. Why not case reports? We would need a proper post-marketing surveillance system to obtain reliable incidence figures. Yet, such a system does not exist. Therefore, the data generated by this paper are next to worthless.
All this article does, is confirm that anaphylactic reactions after BVA are a reality. As the treatment has not been proven to be effective for any condition, its risk/benefit balance turns out to be negative. In other words, we should therefore not use BVA.
Guest post by Derk P. Kooi
Political lobbying is not only restricted to major companies, even quackery lobbies extensively in Dutch politics as well as at a European and global level. The EUROpean Complementary and Alternative Medicine Stakeholder Group (EUROCAM) has been active in Europe for some time. EUROCAM recently attracted attention with a statement on antibiotic resistance during the European Antibiotics Awareness Day.[1] EUROCAM claims that Complementary and Alternative Medicine (CAM) could enhance the immune system and could therefore contribute to the fight against antibiotic resistance. An early study conducted by the anthroposophist Erik Baars was referenced, inter alia. However, this medical claim turns out to be pure nonsense.
EUROCAM regularly publishes so-called ‘position papers’ on the contribution CAM could provide to the European health care system. EUROCAM is currently cautious with its medical claims, and rightly so, as it has seriously overstepped the mark in the past. For example, claims about the efficacy of CAM for infections referred to research by Erik Baars, doctor, anthroposophical healthcare lector at the University of Applied Sciences Leiden and researcher at the Louis Bolk Institute. Baars is an associate of the society due to his misleading statements in his publications on the usefulness of CAM, more specifically of the anthroposophical variant.
Where does this fairly unknown club actually come from, what does it do and how seriously should we take it? Well, EUROCAM is an umbrella organisation for various alternative therapists and their patients. We are talking about Ayurveda, homeopathy, osteopathy, anthroposophy, herbal medicine, traditional (Chinese) medicine, Reiki and acupuncture. The Dutch Registry of Complementary Care Professionals (RBCZ) is also affiliated with EUROCAM. Classical homeopath Annemieke Boelsma is the contact person of the RBCZ at EUROCAM.
It is unclear precisely when EUROCAM was created, the LinkedIn page says 2009. The figurehead of the club is “secretary general” Ton Nicolaï. This homeopathic doctor is also well known to Vereniging tegen de Kwakzalverij, (www.kwakzalverij.nl) the Dutch Society against Quackery. The treasurer of EUROCAM is business administrator Wim Menkveld. Menkveld is on the Advisory Board of the Hortus Botanicus of Leiden. He is also active on the board of the European Patients’ Federation of Homeopathy. EUROCAM thus seems to have originated mainly from Dutch homeopathic circles.
Furthermore, TV producer Miranda Eilert-Ruchtie from Hilversum sits on the EUROCAM board. According to the EUROCAM website, she acts as their “operations manager” and communications advisor. The German Heilprakterin Sonja Maric, an anthropologist and “specialist in Tibetan medicine”, also acts as a communications consultant.
The European Transparency Register shows that in 2020 the total budget of the organisation was 40,498 euros; no more recent data is available. In the year 2018, 5,000 euros were reserved as an honorarium for Mr Nicolaï, for the 0.5 FTE that he works for the organisation. Miranda Eilert-Ruchtie works a number of hours a week for EUROCAM, as a freelancer. Sonja Maric does this on a voluntary basis.
EUROCAM is a member of the European Public Health Alliance (EPHA), the European Union Health Policy Platform. The World Health Organisation (WHO) recognises the organisation as a non-state actor, which means that both the EU and the WHO consider EUROCAM to be a serious legal entity. In the past, EUROCAM has intervened in public EU consultations in the fields of aging, pharmaceutical strategy, cancer, and digital data and services.
EUROCAM provides the secretariat of the MEP Interest Group on Integrative Medicine and Health, a group of five European parliamentarians who have set themselves the goal of promoting integrative medicine at the European level. Co-chairs are Finish Sirpa Pietikäinen, a European parliamentarian for the Christian Democrats, and French Michèle Rivasi, a European parliamentarian for the Greens. The other members are Luxembourg’s Tilly Metz, the Italian Eleonara Evi, and the Danish Margrete Auken. It is noteworthy that they are European parliamentarians for the Greens. They are all members of the European Parliament’s Committee on the Environment, Public Health and Food Safety (ENVI). Eleonara Evi was part of the illustrious Five Star Movement until 2020, known for its anti-vaccination stance. The Member of European Parliament (MEP) Interest Group organises annual events with speakers who are the same people who perform at EUROCAM symposia. These include the aforementioned anthroposophist Erik Baars. Baars worked closely with EUROCAM boss Ton Nicolaï in a European research project on CAM alternatives to antibiotics. More about his bad science later.
The texts EUROCAM produces nowadays (on its website) are carefully written, and the medical claims are carefully formulated. The texts are larded with synonyms for “possible”, known in linguistics as hedging. For example “Several CAM methods have shown high potential to reduce cancer pain”.[2] Generic health claims are also often used to suggest medical benefits, for example in the context of COVID-19, ‘In building and maintaining resistance to infectious illness, CAM modalities as a part of Integrative Medicine & Health can play an important role because they mobilise and stimulate people’s self-regulating capacity, thus increasing their resilience, their immune system.’.[3]
Furthermore, claims are put in the mouths of others, which can be read, for example, in quoting patient expectations: ‘While improving quality of life is the major rationale for CAM use, there is a definite undercurrent of expectation, particularly among the younger patients, that some therapies might have an anticancer effect (prolongation of remission periods) and slow/stagnate tumour growth (prolongation of survival periods), boost the immune system, making it easier to overcome the disease.’.[4]
The educated reader will immediately see through these strategies, but the question is whether the lobbied politicians targeted by EUROCAM understand these subtleties. EUROCAM has not always been so cautious, by the way. In an undated (presumably 2013) interview with the Dutch Association for Classical Homeopathy, “secretary general” Ton Nicolaï made a number of remarkable statements. For example, he claimed at the time that research shows “that for a number of herbal medicines there is a reasonable amount of evidence that scientifically confirms their effectiveness in respiratory infection treatments”. [5] Nicolaï bases his assertion on recent research by Erik Baars conducted as part of a European research programme that aimed to find CAM alternatives to antibiotics.
The report of this project, which ended in 2018, can be found on the EUROCAM website.[6] The authors of this report are, not surprisingly, Erik Baars and Ton Nicolaï. The report contains practically no hard science. Sub-studies focus on, for example, the frequency of antibiotic prescribing among alternative-working GPs and on the best practice of CAM believers. A so-called systematic review of systematic reviews offers good starting points to evaluate Mr Nicolaï’s claim: ‘A systematic review of systematic reviews demonstrates that there are specific, evidence-supported, promising CAM treatments for acute, uncomplicated RTIs [uncomplicated respiratory tract infections, ed.] and that they are safe.’
Here, a medical claim is made, which is weakened by the use of the hedge term “promising”. The conclusion can be summarised with “There would be ‘promising’ CAM treatments for respiratory infections, and they would be safe”. However, surprisingly, the project report does not refer to this “systematic review of systematic reviews”, nor to any of the other concrete results of the project!
Due to the lack of references, we cannot but conclude that the claim is based on a 2019 article by Erik Baars. One of the co-authors is Ton Nicolaï.[7] The article was published in the journal Evidence Based Complementary and Alternative Medicine (EBCAM), which has a shady reputation. Even one of the founders of EBCAM states that the peer-review system is a farce, and therefore the majority of the articles published in it are useless nonsense.[8] In this article, besides a large amount of vagueness about the “worldview differences” between CAM and medicine, systematic reviews are discussed about the effectiveness and safety of CAM treatments. From this systematic review of systematic reviews, it is concluded that there are promising CAM treatments for respiratory, urinary tract and skin infections and that there is even evidence that some CAM treatments are effective for respiratory infections, but what is this based on?
The reviews that were looked at were split into Cochrane and non-Cochrane reviews. Among the Cochrane reviews, there is one that would demonstrate the efficacy of CAM. It is a review on the use of immunostimulants for the prevention of respiratory tract infections in children.[9] Of the 35 studies that were analysed, six involve small molecules, such as isoprinosine, levamisole and pidotimod. In other words, regular medicine, if it turns out to work, but describing it as being experimental would be more appropriate. Baars’ article states that the review also contains herbal medicine. This is somewhat exaggerated: only one of the 35 studies deals with herbs. Of the remaining 28 studies, 25 cover bacterial extracts and three thymus extracts. Again: Baars does not make clear what this has to do with the CAM that EUROCAM represents.
In summary, EUROCAM is a small European lobbying organisation with perhaps some influence at both European and WHO level. One keeps coming across the same names. The organisation is currently using woolly, disguising language to mask medical claims and to fend off criticism. In the past, this was different when EUROCAM, by means of Ton Nicolaï among others, made very reprehensible statements about the role of CAM in (respiratory tract) infections. For the time being, this little club does not seem to pose much of a threat, but European politicians should, of course, ignore this hobby club.
References
1. ‘Improving patient resilience to reduce the need to rely on anti-infection treatment: the role of Integrative Medicine’. EUROCAM. https://cam-europe.eu/statement-on-amr-2021/ (visited on 28 December 2021) 2. EUROCAM. https://cam-europe.eu/contribution-of-cam-for-a-better-health/cam-in-the-context-of-cancer/ (visited on 3 October 2021) 3. EUROCAM. https://cam-europe.eu/contribution-of-cam-for-a-better-health/cam-in-the-context-of-cancer/ (visited on 3 October 2021) 4. EUROCAM. https://cam-europe.eu/contribution-of-cam-for-a-better-health/cam-in-the-context-of-cancer/ (visited on 3 October 2021)
5. Miranda Ruchtie. In gesprek met Ton Nicolaï, CAM integreren in de Europese gezondheidszorg. [In discussion with Ton Nicolaï, integrating CAM into the European health care system]. Nederlandse Vereniging van Klassiek Homeopaten. [Dutch Association of Classical Homeopaths] https://www.nvkh.nl/nieuwsbrieven-nvkh/interview-met-ton-nicolai (visited on 3 October 2021)
6. Erik Baars, et al. Reducing the need for antibiotics, the contribution of Complementary and Alternative Medicine. EUROCAM, 2018. https://cam-europe.eu/wp-content/uploads/2019/01/CAM-AMR-EUROCAM-Post-Conference-Paper-2018.pdf (visited on 3 October 2021)
7. Erik W. Baars et al. The Contribution of Complementary and Alternative Medicine to Reduce Antibiotic Use: A Narrative Review of Health Concepts, Prevention, and Treatment Strategies. Evid. Based Complement. Alternat. Med., 2019:5365608. DOI: 10.1155/2019/5365608
8. Edzard Ernst. “EBCAM: an alt med journal that puzzles me a great deal”, URL: http://edzardernst.com/2016/05/ebcam-an-alt-med-journal-that-puzzles-me-a-great-deal/ (visited on 8 January 2022)
9. B. E. Del-Rio-Navarro, F. J. Espinosa-Rosales, V. Flenady, and J. J. Sienra-Monge, “Cochrane Review: Immunostimulants for preventing respiratory tract infection in children,” Evidence-Based Child Health: A Cochrane Review Journal, 2012, 7 (2), 629–717.
Two million people in UK are estimated to be currently suffering from long COVID, says the Office for National Statistics. Fatigue continues to be the most common symptom – experienced by 55% of those with self-reported long COVID – followed by 32% with shortness of breath, 23% with a cough, and 23% with muscle ache. The problem is only going to increase in the near future. Thus, many people are frantically looking for an effective therapy. Practitioners of so-called alternative medicine (SCAM) are no exception.
This study aimed to evaluate the potential for inhalation of essential oils to improve energy levels among otherwise healthy female survivors of acute COVID-19 who experience a lack of energy more than five months after recovery.
This was a randomized double-blind, placebo-controlled trial to evaluate the potential for inhalation of Longevity™, a proprietary essential oil blend manufactured by Young Living Essential Oils (Lehi, Utah, USA), on energy levels among female survivors of COVID-19 who continue to experience fatigue more than 5 months recovery from the acute infection. Forty women were randomized to two groups: intervention and placebo. The placebo product contained an inert, odorless fractionated coconut oil. Both groups inhaled the assigned product twice daily for fourteen consecutive days. Fatigue scores were measured using the Multidimensional Fatigue Symptom Inventory (MFSI). Secondary outcomes included scores on each of the MFSI’s ten subscales.
Individuals who inhaled the essential oil blend for 2 weeks had significantly lower fatigue scores after controlling for baseline scores, employment status, BMI, olfactory function, and time since diagnosis, with a large effect size (F (1,39) = 6.15, p = .020, partial eta squared = 0.198). Subscale analysis identified subscales of vigor, as well as global, behavioral, general, and mental fatigue as benefiting from the intervention. This study provides evidence that a proprietary aromatherapy blend can significantly improve energy levels among women who are experiencing fatigue after recovering from COVID-19.
The authors concluded that the use of aromatherapy with Longevity™ essential oil blend to boost energy levels in women who have recovered from COVID-19 provides a novel, non-invasive approach to improving quality of life in this population. This intervention is particularly beneficial for global and mental fatigue, as well as vigor. Other subdomains may experience improvements to energy levels with a smaller effect size; future studies should be conducted to explore this potential.
This trial was funded by Young Living Essential Oils. Perhaps, this explains why there is no mention of the elephant in the room: the trial was not blind! Participants in the verum group knew that they received aromatherapy. Likewise, participants in the placebo group knew that they received the placebo.
Could this fact have influenced the outcome? Certainly!
Could the trial have been designed better? Certainly!
All the investigators needed to do is to use a nice-smelling oil that, according to aromatherapists, does not boost energy, as the placebo.
As it stands, we have no idea whether the authors’ assumption that the verum oil caused the effect is true.
Pity!
Or maybe not?
Perhaps Young Living Essential Oils, the sponsor of the study and producer of the oil never wanted to know the truth. Maybe they are happy to abuse science as a marketing tool?
I have written about Bioscan before; for instance here. Now there is more news about the device. In Germany, the manufacturers of Bioscan have been sued and found guilty of fraud.
The two managing directors of the company were sentenced to imprisonment for two and three years respectively and together they have to pay a fine of over 2.5 million euros. The presiding judge considered it proven that the manufacturers had sold useless devices. He said, “A measuring device that measures nothing is about as useful as a car that does not drive.” In addition, a former sales director was sentenced to a fine of 90 daily rates.
The three leading employees of the company were charged with commercial fraud and violations of the Therapeutic Products Advertising Act. The company from Pliezhausen had claimed that their device would measure blood and nutrient values in the body in an uncomplicated way and thus replace a time-consuming laboratory diagnosis.
The Bioscan device consists of two metal rods. You have to take them in your hand, according to the company’s instructions. They would then measure magnetic waves and produce a result. More than 200 medically important health data could allegedly be recorded, for example, cholesterol or testosterone levels. The court had summoned several experts to assess the device. However, they found that the device measured nothing except the current flowing through the cables.
The manufacturers had been doing a huge business with the device for years. The company is said to have earned almost 6 million euros. The devices are still being sold today, for instance, in Austria and Switzerland, among other countries. Despite all the criticism and the court case, the two managing directors had not stopped sales.
________________________
When I googled ‘Bioscan’ yesterday (30/5), the website informed me that:
The BioScan system is an FDA cleared, state of the art testing machine that scans the body’s organs and functions for imbalances using electrodermal screening (EDS).
BioScan SRT
What Is Stress Reduction Testing?
SRT is a remarkable new procedure that combines the disciplines of Acupuncture, Biofeedback and Homeopathy with Laser Light technology. A computerized scan or test is done to see what your body is sensitive to, and how it is out of balance, then help it learn not to be.
Are there any side effects?
No. A small percentage of clients report slight flushing or congestion for a short time (an hour or so) after their session, but this is actually a sign that the body is detoxifying (a good thing)! This process is safe, fast, non-invasive and painless. Unlike skin tests the actual substance is not used, so the body perceives its presence, it as if it were there, but does not act upon it.
What does the BioScan SRT treat?
The BioScan SRT Wellness System does not diagnose or treat any specific condition. Through the use of our FDA-cleared biofeedback technology, the BioScan SRT is able to assess with a very high degree of specificity which substances create increased levels of stress to the body.These specific stress inducing substances are often times what trigger the nervous systems fight or flight reactions which are expressed in a myriad of symptoms that have been scientifically proven to be associated with high levels of stress.
What substances can the BioScan SRT identify as stressors?
The BioScan SRT contains tens of thousands of substances in the main procedure libraries and up to an additional 50,000 substances in the advanced procedure libraries. This technology can identify almost every known substance that could possibly cause a stress reaction.
_________________________
And on the Internet, it takes just a minute to find a Bioscan device for sale. It would set you back by 119.98 Euros.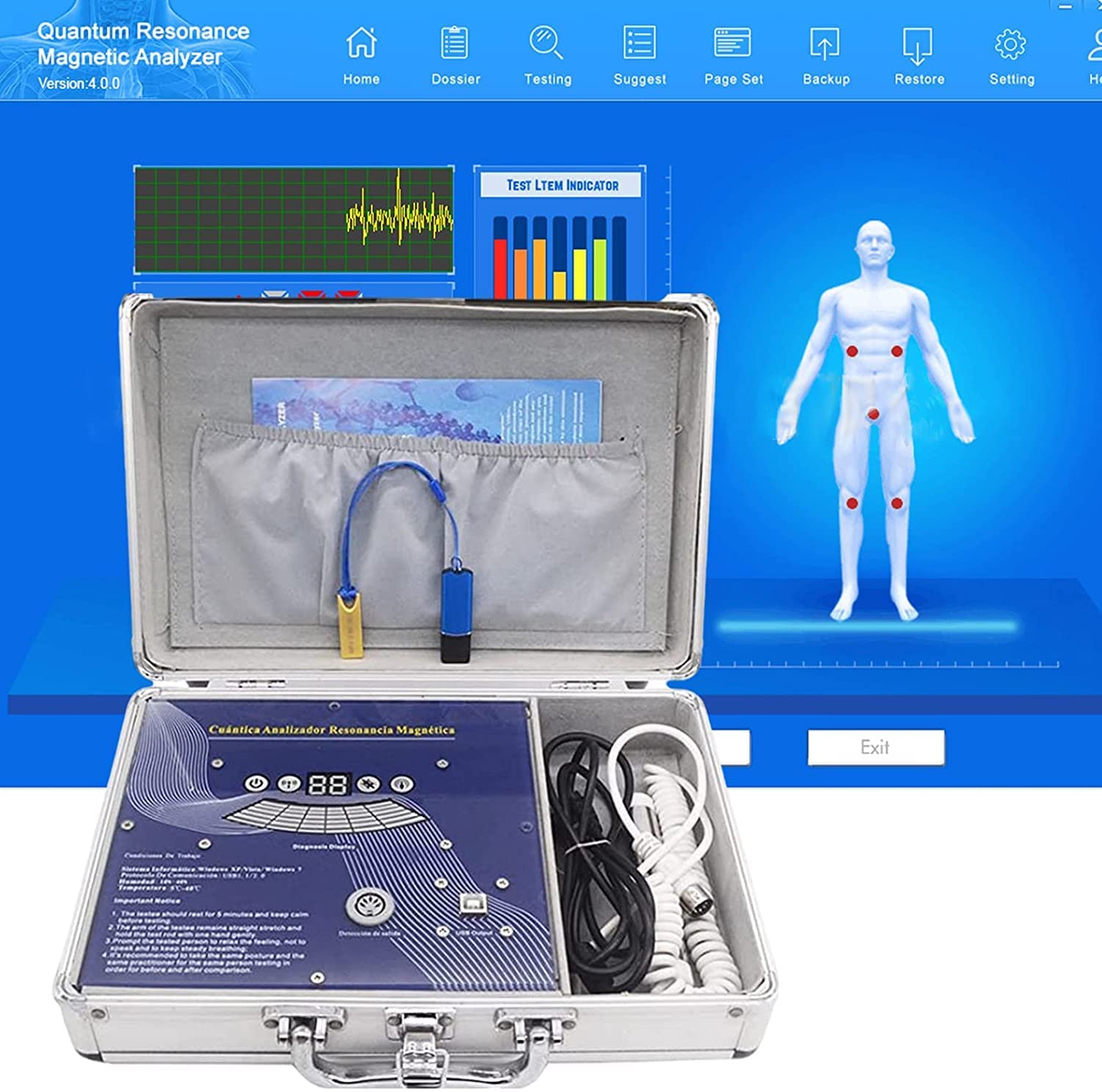
_________________________
Say no more!
Osteopathic visceral manipulation (VM) is a bizarre so-called alternative medicine (SCAM) that has been featured on this blog with some regularity, e.g.:
- Osteopathic visceral manipulation: a new study fails to convince anyone
- Visceral manipulation…you couldn’t make it up
- Intravaginal manipulations by (German) osteopaths: a new low point for clinical research into alternative medicine?
- Visceral osteopathy is implausible and does not work … SO, LET’S FORGET ABOUT IT ONCE AND FOR ALL
Rigorous trials fail to show that it works for anything. So, the obvious solution to this dilemma is to conduct dodgy trials!
This study tested the effects of VM on dysmenorrhea, irregular, delayed, and/or absent menses, and premenstrual symptoms in PCOS patients.
Thirty Egyptian women with polycystic ovary syndrome (PCOS), with menstruation-related complaints and free from systematic diseases and/or adrenal gland abnormalities, participated in a single-blinded, randomized controlled trial. They were recruited from the women’s health outpatient clinic in the faculty of physical therapy at Cairo University, with an age of 20-34 years, and a body mass index (BMI) ≥25, <30 kg/m2. Patients were randomly allocated into two equal groups (15 patients); the control group received a low-calorie diet for 3 months, and the study group that received the same hypocaloric diet added to VM to the pelvic organs and their related structures for eight sessions over 3 months. Evaluations for body weight, BMI, and menstrual problems were done by weight-height scale, and menstruation-domain of Polycystic Ovary Syndrome Health-Related Quality of Life Questionnaire (PCOSQ), respectively, at baseline and after 3 months from interventions. Data were described as mean, standard deviation, range, and percentage whenever applicable.
Of 60 Egyptian women with PCOS, 30 patients were included, with baseline mean age, weight, BMI, and a menstruation domain score of 27.5 ± 2.2 years, 77.7 ± 4.3 kg, 28.6 ± 0.7 kg/m2, and 3.4 ± 1.0, respectively, for the control group, and 26.2 ± 4.7 years, 74.6 ± 3.5 kg, 28.2 ± 1.1 kg/m2, and 2.9 ± 1.0, respectively, for the study group. Out of the 15 patients in the study group, uterine adhesions were found in 14 patients (93.3%), followed by restricted uterine mobility in 13 patients (86.7%), restricted ovarian/broad ligament mobility (9, 60%), and restricted motility (6, 40%). At baseline, there was no significant difference (p>0.05) in any of the demographics (age, height), or dependent variables (weight, BMI, menstruation domain score) among both groups. Post-study, there was a statistically significant reduction (p=0.000) in weight, and BMI mean values for the diet group (71.2 ± 4.2 kg, and 26.4 ± 0.8 kg/m2, respectively) and the diet + VM group (69.2 ± 3.7 kg; 26.1 ± 0.9 kg/m2, respectively). For the improvement in the menstrual complaints, a significant increase (p<0.05) in the menstruation domain mean score was shown in the diet group (3.9 ± 1.0), and the diet + VM group (4.6 ± 0.5). On comparing both groups post-study, there was a statistically significant improvement (p=0.024) in the severity of menstruation-related problems in favor of the diet + VM group.
The authors concluded that VM yielded greater improvement in menstrual pain, irregularities, and premenstrual symptoms in PCOS patients when added to caloric restriction than utilizing the low-calorie diet alone in treating that condition.
WHERE TO START?
- Tiny sample size.
- A trail design (A+B vs B) which will inevitably generate a positive result.
- Questionable ethics.
VM is a relatively invasive and potentially embarrassing intervention for any woman; I imagine that this is all the more true in Egypt. In such circumstances, it is mandatory to ask whether a planned study is ethically justifiable. I would answer this question related to an implausible treatment like VM with a straight NO!
I realize that there may be people who disagree with me. But even those guys should accept that, at the very minimum, such a study must be designed such that it leads to a clear answer – is VM effective or not? The present trial merely suggests that the placebo effect associated with VM is powerful (which is hardly surprising for a therapy like VM).
Acupuncture is often promoted as a therapeutic option for obesity and weight control. The aim of this study was to investigate the effects of electroacupuncture (EA) on body weight, body mass index (BMI), skin fold thickness, waist circumference and skin temperature of the abdominal region in non-obese women with excessive abdominal subcutaneous fat.
A total of 50 women with excessive abdominal subcutaneous fat (and average BMI of 22) were randomly assigned to one of two groups:
- an EA group (n = 25) receiving 10 EA sessions (insertion of needles connected to an electrical stimulator at a frequency of 40 Hz for 40 min),
- a control group (n = 25) that received no treatment.
Outcome measures evaluated included waist circumference, supra-iliac and abdominal skinfolds, body composition and superficial skin temperature (measured by cutaneous thermography) before and after treatment.
Compared with the untreated group, women in the EA group exhibited decreased supra-iliac and abdominal skin folds (p < 0.001), waist circumference (p < 0.001), percentage body fat (p = 0.001) and percentage abdominal fat (p < 0.001). In addition, the EA group showed an elevated skin temperature at the site of the treatment. However, EA did not significantly impact body weight (p = 0.01) or BMI (p = 0.2).
The authors concluded that EA promoted a reduction in abdominal waist circumference, supra-iliac and abdominal skin folds, and percentage body and abdominal fat in women of normal BMI with excessive abdominal subcutaneous fat, as well as an increase in the superficial skin temperature of the abdominal region.
If we did not know that acupuncture researchers were all honest investigators testing hypotheses the best they can, we could almost assume that some are trying to fool us. The set-up of this study is ideally suited to introduce a proper placebo treatment. All one has to do is to not switch on the electrical stimulator in the control group. Why did the researchers not do that? Surely not because they wanted to increase the chances of generating a positive result; that would have been dishonest!!!
So, as it stands, what does the study tell us? I think it shows that, compared to patients who receive no treatment, patients who do receive the ritual of EA are better motivated to adhere to calorie restrictions and dietary advice. Thus, I suggest to re-phrase the conclusions of this trial as follows:
The extra attention of the EA treatment motivated obese patients to eat less which caused a reduction in abdominal waist circumference, supra-iliac and abdominal skin folds, and percentage body and abdominal fat in women of normal BMI with excessive abdominal subcutaneous fat.
I was alerted to the following conference announcement:
The MEP Interest Group on Integrative Medicine and Health is delighted to invite you to the event ‘Integrative Medicine and Health in prevention and management of COVID-19 and long COVID’ on Thursday 2 June 16.00–18.00 CEST.
This event will give you in-depth information about:
Expert speakers will share their knowledge and insights about how:
• Complementary and Integrative Medicine and Health interventions can improve resilience to COVID-19 infection.
• Promoting resilience and health restoration can reduce the risk of severe COVID-19 or development of Long COVID.
• These interventions can improve the recovery from Long COVID.
Key speakers and topics:
Therapeutic strategies of complementary medicines in the COVID 19 pandemic and Long COVID in addition to conventional medicine
Dr Joanna Dietzel, MD Neurologist, Acupuncturist. Department for integrative & complementary medicine, Institute of social medicine, epidemiology and health economics, Charité – Universitätsmedizin Berlin, Germany.
Chinese herbal medicine treatment in cases of infections with SARS-CoV-2 – therapeutic strategies for COVID-19 and Long COVID
Dr Christian Thede, MD, General practitioner, specialised in Acupuncture and Chinese Medicine. Former lecturer in Chinese medicine, University of Witten-Herdecke, Germany
Instructor for Acupuncture and Chinese Medicine at International Society of Chinese Medicine (SMS).
Traditional and Complementary Medicine contributions to health system resilience during COVID-19 – the WHO perspective
Dr Geetha Kopalakrishna, MD, Bachelor of Ayurvedic Medicine & Surgery
Technical Officer at Traditional, Complementary & Integrative Medicine, Department of Service Delivery and Safety, World Health Organization, Geneva, Switzerland
Key member of the AYUSH-based COVID-19 response Task Force for the Government of India.
Research programme into integrative medicine’s contribution to improving resilience to COVID-19 infection and reducing the risk of severe COVID-19 or development of Long COVID
Dr Helene M. Langevin, Director at National Center for Complementary and Integrative Health, National Institutes of Health, Bethesda, Maryland (MD), USA. Previously, Director of the Harvard Osher Center for Integrative Medicine and professor of medicine at Harvard Medical School, Boston (MA) and professor of neurological sciences at the Larner College of Medicine at the University of Vermont (VT).
Q&A sessions after the presentations.
Resilience to infections: a solution for COVID-19 and other infectious illnesses
Studies show that certain common medical conditions put people at higher risk for severe illness and death from COVID-19. Nearly two-thirds of COVID-19 hospitalizations could be attributed to obesity, diabetes, hypertension, and heart failure. There is increasing awareness that a health system that focuses on improving health could prevent all these conditions to a large extent.
Long COVID
More than 40% of people who have or had COVID-19 get long COVID, and among people who needed hospitalization, the statistics go up to 57%. The recovery from such post viral syndromes will be greatly helped by offering patients access to complementary and integrative medicine interventions that aim at restoring their health balance.
MEP Interest Group on Integrative Medicine and Health
The event is hosted by the members of the MEP Interest Group on Integrative Medicine & Health:
Michèle Rivasi, Greens/EFA, France
Sirpa Pietikäinen, EPP, Finland
Tilly Metz, Greens/EFA, Luxembourg
Margrete Auken, Greens/EFA, Denmark
Romana Jerković, S&D, Croatia
Manuela Ripa, Greens/EFA, Germany
I had not been aware of the ‘MEP Interest Group on Integrative Medicine & Health‘. Therefore, I looked it up and found this:
The newly established Interest Group on Integrative Medicine & Health continues the work of the former MEP Interest Group on CAM. This group brings together MEPs who work collectively to promote the inclusion of CAM as part of Integrative Medicine & Health in all possible European Parliament public health policy.
Why an Interest Group in the European Parliament?
One in two EU citizens uses complementary medicine either alongside or as an alternative to conventional biomedical care. This high demand is not yet reflected in EU or national health policy or provision. In addition, there is diversity in complementary medicine regulation across the EU. There are differences in who can practice complementary medicine, what qualifications are required and how services are offered and financed. These discrepancies mean that citizens experience practical and attitudinal barriers that limit their access to and use of TCIM.
The health sector in the EU Member States is facing considerable challenges, such as antimicrobial resistance (AMR), increasing prevalence of Non-Communicable Diseases (NCDs) and soaring costs. Complementary medicine can offer a significant contribution to meet these challenges. These modalities are “integrative”, offering patient-centered healthcare, based on evidence-informed integration of conventional biomedicine and complementary medicine. Integrative Medicine and Health focuses on the whole person and considers the individual in its physical, psychological, spiritual, social and environmental context. It is inclusive of all professions and practices that use this approach and meets the demand of EU citizens for a more holistic, patient-centered approach in medicine. At the same time, TCIM is at the center of political and scientific debate. In this context, a forum for discussion on Integrative and Complementary Medicine’s contribution to EU health systems will bring clarity and rationality to this debate.
Aims and objectives of the Interest Group on Integrative Medicine & Health
- Establish and maintain a forum for discussion and action with all stakeholders regarding Integrative Medicine and Health.
- Raise awareness of Integrative Medicine and its contribution to more sustainable healthcare systems in the EU and a more holistic approach to health.
- Focus on the integration of complementary modalities into the health systems of the EU Member States.
- Protect and promote citizens’ right to choose their own healthcare while providing access to Integrative Medicine and Health information.
- Advocate for EU involvement in setting unified standards to regulation of Integrative Medicine and Health.
__________________________________
Unified standards? But what about high or perhaps just scientific standards? What about first doing the research and then making claims about CAM or TCIM or however you decide to call it? Has common sense gone out of fashion?
Yes, you guessed it: I am seriously underwhelmed by all this. To show you why, let me list just a few claims from the above two statements that are based purely on wishful thinking:
- Complementary and Integrative Medicine and Health interventions can improve resilience to COVID-19 infection.
- These interventions can improve the recovery from Long COVID.
- Studies show that certain common medical conditions put people at higher risk for severe illness and death from COVID-19.
- The recovery from such post viral syndromes will be greatly helped by offering patients access to complementary and integrative medicine interventions that aim at restoring their health balance.
- One in two EU citizens uses complementary medicine either alongside or as an alternative to conventional biomedical care.
- The health sector in the EU Member States is facing considerable challenges, such as antimicrobial resistance (AMR), increasing prevalence of Non-Communicable Diseases (NCDs) and soaring costs. Complementary medicine can offer a significant contribution to meet these challenges.
- These modalities are “integrative”, offering patient-centered healthcare, based on evidence-informed integration of conventional biomedicine and complementary medicine.
- Integrative medicine … meets the demand of EU citizens for a more holistic, patient-centered approach in medicine.
I find all this confusing and concerning in equal measure. I also seriously doubt that the forum for discussion on Integrative and Complementary Medicine will bring clarity and rationality to this debate. If they really wanted a debate, they would need to include a few critical thinkers; can anyone recognize one on the list of speakers? I cannot!
I fear the aim of the group and their meeting is to mislead us all into thinking that CAM, TCIM, etc. generate more good than harm without ever delivering the evidence for that assumption. Therefore, I suggest they rename both the conference as well as their group:
‘Wishful thinking in prevention and management of COVID-19 and long COVID’
and
MEP Interest Group on Wishful Thinking and Promotion of Quackery
PS
As an antidote to wishful thinking, I recommend reading some proper science papers on the subject. Here are the conclusions of an up-to-date and wishful-thinking-free review on the subject of post-acute infection syndrome:
Unexplained post-acute infection syndromes (PAISs) appear to be an under-recognized feature of a spectrum of infectious diseases in a minority of patients. At present, our understanding of the underlying pathophysiologic mechanisms and etiologic factors is poor and there are no known objective markers or effective therapeutic options. More basic biomedical research is needed. The overlap of symptoms, signs, and general features of the individual PAISs suggests the involvement of shared pathological pathways and the possibility that common diagnostic markers, or even a unified etiological model, might be established.
However, some symptoms or clinical characteristics seem to be trigger-specific or more prevalent in one PAIS than in others, emphasizing the need for cohorts with a well-documented infectious trigger. The overall clinical picture of many PAISs often overlaps with the presentation of post-infectious ME/CFS or fibromyalgia, or resembles other fatiguing, neurological, or rheumatic disorders. Exploiting existing knowledge of these conditions might help guide future scientific discovery and progress in clinical care.
The SARS-CoV-2 pandemic uncovered a significant gap in knowledge about post-acute sequelae of infectious diseases and identified the need for better diagnostic care and clinical infrastructure for patients experiencing these long-term effects. In addition to basic biomedical research, more needs to be done to refine diagnostic criteria and obtain more reliable estimates of the prevalence and societal burden of these disorders to help shape health-policy decisions. Moreover, we call for unified nomenclature and better conceptualization of post-acute infection symptoms.
There is much to be done, but the unprecedented amount of attention and resources that have recently been allocated to the study of COVID-19-related pathology brings a promise of much-needed progress in the wider field of unexplained infection-associated chronic disability.
Ayush-64 is an Ayurvedic formulation, developed by the Central Council for Research in Ayurvedic Sciences (CCRAS), the apex body for research in Ayurveda under the Ministry of Ayush. Originally developed in 1980 for the management of Malaria, this drug has now been repurposed for COVID-19 as its ingredients showed notable antiviral, immune-modulator, and antipyretic properties. Its ingredients are:
| Alstonia scholaris R. Br. Aqueous extract of (Saptaparna) | Bark-1 part |
| Picrorhiza Kurroa Royle Aqueous extract of (Kutki) | Rhizome-1 part |
| Swertia chirata Buch-Ham. Aqueous extract of (Chirata) | Whole plant-1 part |
| Caesalphinia crista, Linn. Fine powder of seed (Kuberaksha) | Pulp-2 parts |
The crucial question, of course, is does AYUSH-64 work?
An open-label randomized controlled parallel-group trial was conducted at a designated COVID care centre in India with 80 patients diagnosed with mild to moderate COVID-19 and randomized into two groups. Participants in the AYUSH-64 add-on group (AG) received AYUSH-64 two tablets (500 mg each) three times a day for 30 days along with standard conventional care. The control group (CG) received standard care alone.
The outcome measures were:
- the proportion of participants who attained clinical recovery on days 7, 15, 23, and 30,
- the proportion of participants with negative RT-PCR assay for COVID-19 at each weekly time point,
- change in pro-inflammatory markers,
- metabolic functions,
- HRCT chest (CO-RADS category),
- the incidence of Adverse Drug Reaction (ADR)/Adverse Event (AE).
Out of 80 participants, 74 (37 in each group) contributed to the final analysis. A significant difference was observed in clinical recovery in the AG (p < 0.001 ) compared to CG. The mean duration for clinical recovery in AG (5.8 ± 2.67 days) was significantly less compared to CG (10.0 ± 4.06 days). Significant improvement in HRCT chest was observed in AG (p = 0.031) unlike in CG (p = 0.210). No ADR/SAE was observed or reported in AG.
The authors concluded that AYUSH-64 as adjunct to standard care is safe and effective in hastening clinical recovery in mild to moderate COVID-19. The efficacy may be further validated by larger multi-center double-blind trials.
I do object to these conclusions for several reasons:
- The study cannot possibly determine the safety of AYUSH-64.
- Even for assessing its efficacy, it was too small.
- The trial design followed the often-discussed A+B vs B concept and is thus prone to generate false-positive results.
I believe that it is highly irresponsible, during a medical crisis like ours, to conduct studies that can only produce unreliable findings. If there is a real possibility that a therapy might work, we do need to test it, but we should take great care that the test is rigorous enough to generate reliable results. This, I think, is all the more true, if – like in the present case – the study was done with governmental support.

