progress
Is acupuncture more than a theatrical placebo? Acupuncture fans are convinced that the answer to this question is YES. Perhaps this paper will make them think again.
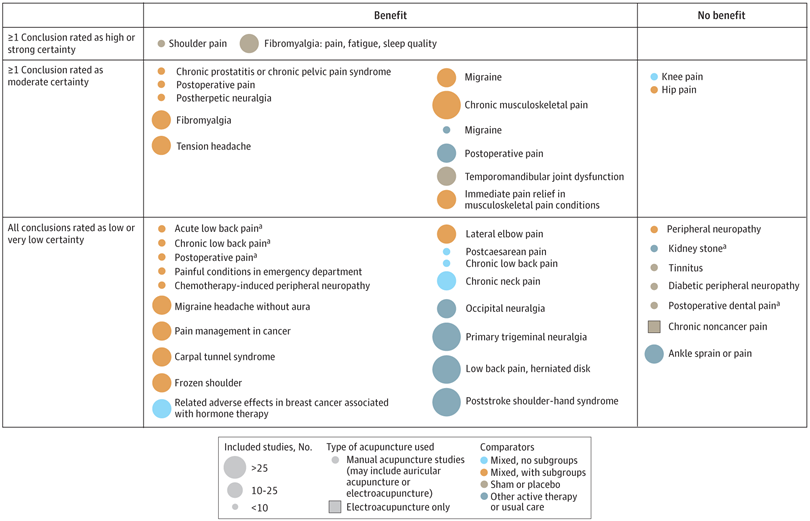
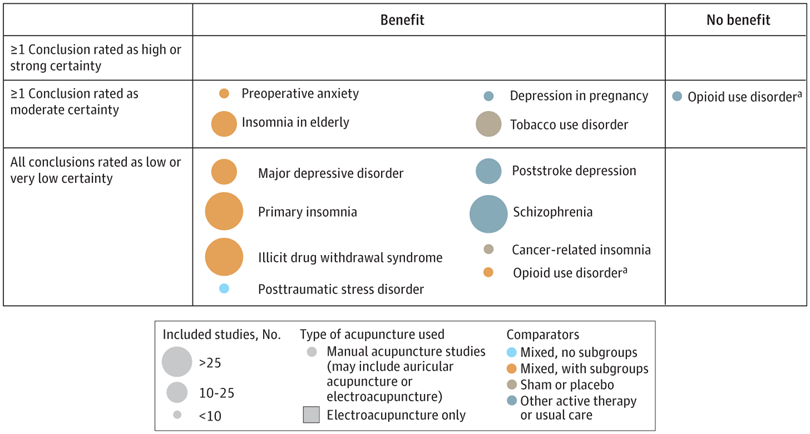
A new analysis mapped the systematic reviews, conclusions, and certainty or quality of evidence for outcomes of acupuncture as a treatment for adult health conditions. Computerized search of PubMed and 4 other databases from 2013 to 2021. Systematic reviews of acupuncture (whole body, auricular, or electroacupuncture) for adult health conditions that formally rated the certainty, quality, or strength of evidence for conclusions. Studies of acupressure, fire acupuncture, laser acupuncture, or traditional Chinese medicine without mention of acupuncture were excluded. Health condition, number of included studies, type of acupuncture, type of comparison group, conclusions, and certainty or quality of evidence. Reviews with at least 1 conclusion rated as high-certainty evidence, reviews with at least 1 conclusion rated as moderate-certainty evidence and reviews with all conclusions rated as low- or very low-certainty evidence; full list of all conclusions and certainty of evidence.
A total of 434 systematic reviews of acupuncture for adult health conditions were found; of these, 127 reviews used a formal method to rate the certainty or quality of evidence of their conclusions, and 82 reviews were mapped, covering 56 health conditions. Across these, there were 4 conclusions that were rated as high-certainty evidence and 31 conclusions that were rated as moderate-certainty evidence. All remaining conclusions (>60) were rated as low- or very low-certainty evidence. Approximately 10% of conclusions rated as high or moderate-certainty were that acupuncture was no better than the comparator treatment, and approximately 75% of high- or moderate-certainty evidence conclusions were about acupuncture compared with a sham or no treatment.
Three evidence maps (pain, mental conditions, and other conditions) are shown below
The authors concluded that despite a vast number of randomized trials, systematic reviews of acupuncture for adult health conditions have rated only a minority of conclusions as high- or moderate-certainty evidence, and most of these were about comparisons with sham treatment or had conclusions of no benefit of acupuncture. Conclusions with moderate or high-certainty evidence that acupuncture is superior to other active therapies were rare.
These findings are sobering for those who had hoped that acupuncture might be effective for a range of conditions. Despite the fact that, during recent years, there have been numerous systematic reviews, the evidence remains negative or flimsy. As 34 reviews originate from China, and as we know about the notorious unreliability of Chinese acupuncture research, this overall result is probably even more negative than the authors make it out to be.
Considering such findings, some people (including the authors of this analysis) feel that we now need more and better acupuncture trials. Yet I wonder whether this is the right approach. Would it not be better to call it a day, concede that acupuncture generates no or only relatively minor effects, and focus our efforts on more promising subjects?
I know, I have often posted nasty things about integrative medicine and those who promote it. Today, I want to make good for all my sins and look at the bright side.
Imagine you are a person convinced of the good that comes from so-called alternative medicine (SCAM). Imagine you believe it has stood the test of time, is natural, holistic, tackles the root problems of illness, etc., etc. Imagine you are such a person.
Your convictions made you support more research into SCAM because you feel that evidence is needed for it to be more generally accepted. So, you are keen to see more studies proving the efficacy of this or that SCAM in the management of this or that condition.
This, unfortunately, is where the problems start.
Not only is there not a lot of money and even fewer scientists to do this research, but the amount of studies that would need doing is monstrously big:
- There are hundreds of different types of SCAM.
- Each SCAM is advocated for hundreds of conditions.
Consequently, tens of thousands of studies are needed to only have one trial for each specific research question. This is tough for a SCAM enthusiast! It means he/she has to wait decades to see the light at the end of the tunnel.
But then it gets worse – much worse!
As the results of these studies come in, one after the other, you realize that most of them are not at all what you have been counting on. Many can be criticized for being of dismal quality and therefore inconclusive, and those that are rigorous tend to be negative.
Bloody hell! There you have been waiting patiently for decades and now you must realize that this wait did not take you anywhere near the goal that was so clear in your sight. Most reasonable people would give up at this stage; they would conclude that SCAM is a pipedream and direct their attention to something else. But not you! You are single-minded and convinced that SCAM is the future. Some people might even call you obsessed – obsessed and desperate.
It is out of this sense of desperation that the idea of integrative medicine was born. It is a brilliant coup that solves most of the insurmountable problems outlined above. All you need to do is to take the few positive findings that did emerge from the previous decades of research, find a political platform, and loudly proclaim:
SCAM does work.
Consumers like SCAM.
SCAM must be made available to all.
Consumers deserve the best of both worlds.
The future of healthcare evidently lies in integrated medicine.
Forgotten are all those irritating questions about the efficacy of this or that treatment. Now, it’s all about the big issue of wholesale integration of SCAM. Forgotten is the need for evidence – after all, we had decades of that! – now, the issue is no longer scientific, it is political.
And if anyone has the audacity to ask about evidence, he/she can be branded as a boring nit-picker. And if anyone doubts the value of integrated medicine, he/she will be identified as a politically incorrect dinosaur.
Mission accomplished!
Today, you cannot read a newspaper or listen to the radio without learning that there has been a significant, sensational, momentous, unprecedented, etc. breakthrough in the treatment of Alzheimer’s disease. The reason for all this excitement (or is it hype?) is this study just out in the NEJM:
BACKGROUND
The accumulation of soluble and insoluble aggregated amyloid-beta (Aβ) may initiate or potentiate pathologic processes in Alzheimer’s disease. Lecanemab, a humanized IgG1 monoclonal antibody that binds with high affinity to Aβ soluble protofibrils, is being tested in persons with early Alzheimer’s disease.
METHODS
We conducted an 18-month, multicenter, double-blind, phase 3 trial involving persons 50 to 90 years of age with early Alzheimer’s disease (mild cognitive impairment or mild dementia due to Alzheimer’s disease) with evidence of amyloid on positron-emission tomography (PET) or by cerebrospinal fluid testing. Participants were randomly assigned in a 1:1 ratio to receive intravenous lecanemab (10 mg per kilogram of body weight every 2 weeks) or placebo. The primary end point was the change from baseline at 18 months in the score on the Clinical Dementia Rating–Sum of Boxes (CDR-SB; range, 0 to 18, with higher scores indicating greater impairment). Key secondary end points were the change in amyloid burden on PET, the score on the 14-item cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-cog14; range, 0 to 90; higher scores indicate greater impairment), the Alzheimer’s Disease Composite Score (ADCOMS; range, 0 to 1.97; higher scores indicate greater impairment), and the score on the Alzheimer’s Disease Cooperative Study–Activities of Daily Living Scale for Mild Cognitive Impairment (ADCS-MCI-ADL; range, 0 to 53; lower scores indicate greater impairment).
RESULTS
A total of 1795 participants were enrolled, with 898 assigned to receive lecanemab and 897 to receive placebo. The mean CDR-SB score at baseline was approximately 3.2 in both groups. The adjusted least-squares mean change from baseline at 18 months was 1.21 with lecanemab and 1.66 with placebo (difference, −0.45; 95% confidence interval [CI], −0.67 to −0.23; P<0.001). In a substudy involving 698 participants, there were greater reductions in brain amyloid burden with lecanemab than with placebo (difference, −59.1 centiloids; 95% CI, −62.6 to −55.6). Other mean differences between the two groups in the change from baseline favoring lecanemab were as follows: for the ADAS-cog14 score, −1.44 (95% CI, −2.27 to −0.61; P<0.001); for the ADCOMS, −0.050 (95% CI, −0.074 to −0.027; P<0.001); and for the ADCS-MCI-ADL score, 2.0 (95% CI, 1.2 to 2.8; P<0.001). Lecanemab resulted in infusion-related reactions in 26.4% of the participants and amyloid-related imaging abnormalities with edema or effusions in 12.6%.
CONCLUSIONS
Lecanemab reduced markers of amyloid in early Alzheimer’s disease and resulted in moderately less decline on measures of cognition and function than placebo at 18 months but was associated with adverse events. Longer trials are warranted to determine the efficacy and safety of lecanemab in early Alzheimer’s disease. (Funded by Eisai and Biogen; Clarity AD ClinicalTrials.gov number, NCT03887455. opens in new tab.)
It’s a good study, and I (like everyone else) hope that it will mean tangible progress in the management of that devastating disease. Most media outlets are announcing the news with the claim that it is the FIRST TIME that any treatment has been shown to delay the cognitive decline of Alzheimer’s disease patients.
But is this true?
I think not!
There have been several studies showing that the herbal remedy GINKGO BILOBA slows the Alzheimer-related decline. Here is the latest systematic review of the subject:
Background: Ginkgo biloba is a natural medicine used for cognitive impairment and Alzheimer’s disease. The objective of this review is to explore the effectiveness and safety of Ginkgo biloba in treating mild cognitive impairment and Alzheimer’s disease.
Methods: Electronic search was conducted from PubMed, Cochrane Library, and four major Chinese databases from their inception up to 1(st) December, 2014 for randomized clinical trials on Ginkgo biloba in treating mild cognitive impairment or Alzheimer’s disease. Meta-analyses were performed by RevMan 5.2 software.
Results: 21 trials with 2608 patients met the inclusion criteria. The general methodological quality of included trials was moderate to poor. Compared with conventional medicine alone, Ginkgo biboba in combination with conventional medicine was superior in improving Mini-Mental State Examination (MMSE) scores at 24 weeks for patients with Alzheimer’s disease (MD 2.39, 95% CI 1.28 to 3.50, P<0.0001) and mild cognitive impairment (MD 1.90, 95% CI 1.41 to 2.39, P<0.00001), and Activity of Daily Living (ADL) scores at 24 weeks for Alzheimer’s disease (MD -3.72, 95% CI -5.68 to -1.76, P=0.0002). When compared with placebo or conventional medicine in individual trials, Ginkgo biboba demonstrated similar but inconsistent findings. Adverse events were mild.
Conclusion: Ginkgo biloba is potentially beneficial for the improvement of cognitive function, activities of daily living, and global clinical assessment in patients with mild cognitive impairment or Alzheimer’s disease. However, due to limited sample size, inconsistent findings and methodological quality of included trials, more research are warranted to confirm the effectiveness and safety of ginkgo biloba in treating mild cognitive impairment and Alzheimer’s disease.
I know, the science is not nearly as good as that of the NEJM trial. I also know that the trial data for ginkgo biloba are not uniformly positive. And I know that, after several studies showed good results, later trials tended not to confirm them.
But this is what very often happens in clinical research: studies are initially promising, only to be disappointing as more studies emerge. I sincerely hope that this will not happen with the new drug ‘Lecanemab’ and that today’s excitement will not turn out to be hype.
The AMA has recently published a short article that – even though not addressing so-called alternative medicine (SCAM) directly – has considerable relevance for the field:
It’s increasingly common for patients to encounter nonphysician practitioners as members of their health care teams. Meanwhile, ever more nonphysician practitioners have received advanced training resulting in a doctorate degree, such as the doctor of nursing practice.
To help patients keep pace with these changes, physicians should make new strides to clarify their roles and credentials vis-a-vis other members of the health care team and also promote collaboration among all health professionals, according to an AMA Council on Ethical and Judicial Affairs report that was adopted at the 2022 AMA Interim Meeting.
The core issue is that “the skill sets and experience of nonphysician practitioners are not the same as those of physicians.” Thus, when nonphysician practitioners identify themselves as “doctors”—consistent with the doctoral-level degrees they earned—“it may create confusion and be misleading to patients and other practitioners,” says the report.
In fact, surveys (PDF) performed as part of the AMA Truth in Advertising Campaign have found that while patients strongly support physician-led health care teams, many are confused about the level of education and training of health professionals—and the confusion isn’t limited to nonphysician practitioners who hold doctorates. For example, roughly one-fifth of respondents think psychiatrists are not physicians, while a similar number think nurse practitioners are physicians.
The AMA Code of Medical Ethics touches on this issue in an opinion on collaborative care, which provides guidance on the roles of physicians in team-based settings where a mix of health professionals provide care.
In SCAM, we have the problem that practitioners often call themselves doctors or physicians without having a medical degree. This confuses patients who might consult and trust these practitioners assuming they have studied medicine. We recently discussed the case of a naturopath who called himself a doctor and failed to diagnose a rectal tumor of his patient. Much more dramatic was the case of a UK-based chiropractor who called herself a doctor, thus attracting a patient suffering from complex health issues contraindicating spinal manipulations. She nonetheless manipulated his neck and promptly killed him.
I know that patients are being misled every day by SCAM practitioners (ab)using the ‘Dr.’ title. Therefore, the AMA reminder is an important, timely, and necessary lesson for SCAM. I feel that the professional organizations of SCAM providers should issue similar reminders to their members and make sure they behave appropriately.
Prof. Harald Walach and his work have been regular topics on this blog (e.g. here, here, and here). Walach has served as the editor of Forschende Komplementärmedizin / Research in Complementary Medicine for 20 years and is now retiring from this post. On this occasion, he just published an EDITORIAL looking both back and ahead on research into so-called alternative medicine (SCAM). Here are the last paragraphs of his piece:
What lies in store? We do not know. “Hidden is the future before me, I am wondering what my destiny will bring,” sings Lensky in Tschaikowsky’s opera Eugen Onegin, and this is a good description of our current situation, not only in medicine, but also politically. If I have one wish for the future of CAM, for the future of our journal, then it is to keep the fire ablaze and uphold the hope of change that has been at the source of its founding and is still empowering many in the field. The field of medicine, but also the world, needs examples of visions and visionaries. The landscape will change. While the beginning of the field and the journal was a decidedly German-speaking, central European enterprise, we have now seen the extension of the field.
China has entered the scene with enormous manpower, a venerable tradition, and a huge amount of experience, research, and funding. Other countries, Iran for instance, are discovering the sources of traditional medical approaches. It might well be the case that those who forget that the world does not end at the rim of the Mediterranean and of the Atlantic will be left behind. It has always been a decisive element of CAM research that it bridged countries, nations, polities, and worldviews. The ISCMR, Consortium, and European Congress for Integrative Medicine (ECIM) conferences probably had as attendees more researchers from outside Europe and the US than from their host countries. Africa is only slowly beginning to enter the scene. The future will be less Euro- and Western-centric than the beginning of CAM, I am quite sure. The Western model of healthcare and economic growth through single pharmacological inventions is not sustainable worldwide and in the long run, apart from the fact that it is conceptually ill-founded. Thus, our hope very likely lies in broadening our view: thinking about other systems of medicine, other approaches, whole-systems thinking. This is actually very similar to our beginning. Every end is a beginning, every beginning is an end, Oscar Wilde used to say.
Apart from the abundant use of platitudes, there are several statements that might deserve a comment:
- The beginning of the field and the journal was a decidedly German-speaking, central European enterprise. Yes, the journal started as a predominantly German publication, yet the field was never mostly German/ central European. SCAM always included many modalities that originated from China, the US, and other non-European countries. Neither was the research into these areas ever dominated by German-speaking investigators.
- China has entered the scene with enormous manpower, a venerable tradition, and a huge amount of experience, research, and funding. This is true – but is it a good development? On this blog, I have often written about the fact that research from China is notoriously unreliable or even fabricated. As the quantity of such work is about to totally overwhelm SCAM research, this is a most concerning development, in my view.
- It has always been a decisive element of CAM research that it bridged countries, nations, polities, and worldviews. I would say that this is not something that characterizes SCAM research. It is a hallmark of any research. And considering my last point, it might soon no longer apply to SCAM. As we are being flooded with unreliable Chinese SCAM research, Chinese dominance might soon stifle criticism of SCAM.
- The Western model of healthcare and economic growth? As far as I can see, the model of economic growth is fast being adopted by non-Western counties.
So, what is the future of SCAM and SCAM research? Like Walach, I don’t know. But contrary to Walach, I hope for something entirely different. I hope that the stupidly short-sighted notion of two types of research and two types of healthcare can eventually be abandoned. In the end, there can only be one type of science – the one that understands itself as critically testing hypotheses by trying to prove them wrong – and only one type of medicine – the one that does more good than harm.
It has been reported that the Regional Court of Dortmund has prohibited the manufacturer of the homeopathic cold remedy Meditonsin from advertising with false health claims. The court did not see sufficient evidence for the advertising claims.
The Consumer Advice Centre (VZ) of North Rhine-Westphalia issued a warning to the Meditonsin manufacturer (MEDICE Arzneimittel Pütter GmbH & Co.) for misleading advertising statements and sued them. The complaint was:
- that the advertising gave the false impression that an improvement in health could be expected with certainty after taking the product,
- that no side effects were to be expected,
- that the product was superior to “chemical-synthetic medicines”.
The Dortmund Regional Court was not convinced by a study referred to by the manufacturer. On its website, the manufacturer of Meditonsin presents the results of a “current, large-scale user study with more than 1,000 patients” under the heading “Proven efficacy & tolerability”. According to a pie chart, 90% of the patients were satisfied or very satisfied with the effect of Meditonsin.
However, according to the VZ, the study was only a “pharmacy-based observational study” with little scientific validity. Despite the lack of evidence, the manufacturer claimed that “the good efficacy and tolerability of Meditonsin® Drops could once again be impressively confirmed”. The Dortmund Regional Court, however, followed the VZ’s statement of grounds for action. “It is not allowed to advertise with statements that give the false impression that a successful treatment can be expected with certainty, as the advertisement for Meditonsin drops suggests,” emphasized Gesa Schölgens, head of “Faktencheck Gesundheitswerbung”, a joint project of the consumer centres of North Rhine-Westphalia and Rhineland-Palatinate. According to the Therapeutic Products Advertising Act, this is prohibited.
The Dortmund Regional Court also found that consumers were misled by the advertising because it gave a false impression that no harmful side effects were to be expected when taking Meditonsin. The package leaflet of the drug listed several side effects. According to this, there could even be an initial worsening of the symptoms after taking the medicine.
According to the VZ, the alleged advantage of the “natural medicinal product” over “many chemical-synthetic medicinal products that only suppress the symptoms”, as presented by the manufacturer, is also inadmissible. This is because it is not permissible to advertise to consumers with claims that the effect is equivalent or superior to that of another medicinal product. This, too, was confirmed by the court.
_________________________
In case you like to know more about the remedy, this is from its English language site:
Meditonsin consists of Aconitum, Atropinum Sulfuricum, Mercurius Cyanatus. Active ingredient is the part of the drug or medicine which is biologically active. This portion of the drug is responsible for the main action of the drug which is intended to cure or reduce the symptom or disease.
Quackery is rife in India. On this blog, I have occasionally reported on this situation, e.g.:
- The new ‘WHO Global Centre for Traditional Medicine’ in India
- Mucormycosis (black fungus): is the Indian AYUSH ministry trying to decimate the population?
- Homeopathy, COVID, India and Prince Charles: not a good mixture!
- Has homeopathy caused the dramatic decline of COVID-19 cases in India?
- Homeopathy research from India is far from trustworthy, and today I can show you why
- Brazil and India collaborate in the promotion of quackery
- Taking the piss again? The story of urine therapy in India
- The intriguing case of homeopathy in India
- Prince Charles’ advocacy of quackery is by no means harmless
- Patient Dies After Homeopath Gives Wrong Injection
- Herbal remedies are good for you … except for the ones that injure your liver
- The ‘AYUSH COVID-19 Helpline’: have they gone bonkers?
Now the Chief Justice of India (CJI) NV Ramana has pointed out that legislation needs to be brought in to save people “from falling prey to fraudulent practices in the name of treatment”. Speaking at the inaugural National Academy of Medical Sciences on ‘Law and Medicine’, the CJI said: “Quackery is the biggest disease affecting India” and that hospitals are “being run like companies, where profit-making is more important than service to society”. The CJI added, “another side of lack of accessible healthcare is giving space to quacks. Quackery begins where awareness ends. Where there is room for myths, there is room for quackery”. He continued, “Owing to the financial and time constraints, a huge majority of the Indian population approaches these untrained and uncertified doctors. Lack of awareness and knowledge, misplaced belief, and sheer inaccessibility have massive ramifications on the health of the country, particularly the rural and underprivileged Indian … The need of the hour is to bring in legislation to save people from falling prey to fraudulent practices in the name of treatment … Private hospitals are being opened at an exponential rate. This is not necessarily a bad thing, but there is a glaring need for balance. We are seeing hospitals being run like companies, where profit-making is more important than service to society.”
I am sure the CJI is correct; India does have a quackery problem. If nothing else, the fact that one website lists a total of 746 Alternative Medicine Colleges in India, leaves little doubt about it.
A review conducted in 2015 reported community pharmacists are willing to adopt a professional role in counselling consumers about the appropriate and safe use of so-called alternative medicine (SCAM) but faced multiple barriers in doing so. This current review aimed to update and extend these findings, by identifying studies published since 2015 that reported on pharmacists across any setting.
Eligible studies published between January 01, 2016, and December 31, 2021, were identified across 6 databases (PubMed, Scopus, Web of Science, EMBASE, ScienceDirect and MEDLINE). A grounded theory approach was used to thematically synthesize the data extracted.
A total of 64studies representing pharmacists across 30 countries were included for review. The study designs varied and included:
- cross-sectional surveys (n = 36),
- qualitative studies (n = 14),
- pseudo-patient studies (n = 3).
Eight studies reported on practice and/or bioethical responsibilities and 19 studies documented factors that would enable pharmacists to fulfill these responsibilities, while 37 studies reported on both.
The authors concluded that these findings indicate research about pharmacists’ responsibilities associated with SCAM is evolving from gap analysis towards research that is proactive in advocating for change in multiple areas. These findings can be used to inform a consensus discussion among pharmacists and key stakeholders regarding a set of professional responsibilities that would serve in the development of: a clearly defined role and associated practice standards, and competency requirements that inform educational learning objectives for inclusion in undergraduate, post-graduate and continuing professional pharmacy education.
I am puzzled why so many researchers in this specific area seem to avoid clearer language plainly stating the essential, simple, and undeniable facts. I am equally puzzled why so few pharmacists speak out.
It is obvious that community pharmacists are firstly healthcare professionals and only secondly shopkeepers. As such, they have important professional and ethical duties. Foremost, they are obliged to inform their customers responsibly – and responsible means telling them about the evidence for or against the SCAM product they are about to purchase. This duty also entails that pharmacists must inform themselves about the best current evidence. In turn, this means they must stop tolerating the current plethora of under- or post-graduate SCAM courses that are not evidence-based.
As we have discussed ad nauseam on this blog, none of this is actually happening (except in very few laudable cases)!
By and large, pharmacists continue to go along with the double standards of a) evidence for conventional drugs and b) fairy tales for SCAM. In the interest of progress, patient safety, and public health, it is time that pharmacists wake up and remind themselves that they are not commercially orientated shopkeepers but ethical healthcare professionals.
I just got this email with sad news: Ken Frazier “died peacefully this morning, three weeks after being diagnosed with acute myeloid leukemia. Judy and I were fortunate to spend time with Ken and Ruth last week and tell Ken personally how much he has meant to us over our entire lives.”
Ken was a part of CFI history quite literally from Day One. In May 1976, writing for Science News, Ken reported on the formation of the Committee for the Scientific Investigation of Claims of the Paranormal (CSICOP). In 1977, Ken joined CSICOP to serve as editor of The Zetetic, which became Skeptical Inquirer in 1978. He held that position ever since, spending the better part of five decades defining and steering the work of the skeptical community in combatting disinformation and pseudoscience. Ken has also published numerous papers and books, e.g.:
Ken’s book Science Under Siege: Defending Science, Exposing Pseudoscience was featured by Science News for its “engaging, insightful, and often surprising essays by researchers and journalists” about “what science is and is not, and what happens when the facts get twisted.” And he was working on yet another book; only a few months ago he wrote to me taking me for help with it:
… I am completing [ a book] on science and pseudoscience, titled Shadows of Science. It was just accepted by Prometheus Books for publication in Fall 2023. I am now working on a final chapter on “Pseudomedicine,” pseudoscience in medicine. This is not my area of expertise so I am relying on many medical professionals who have investigated and written about medical pseudoscience, most prominently you.
My chapter is mainly concerned with broad points and principles in identifying and describing pseudoscience in medicine — SCAM.
I merely ask if you mind if I quote from and paraphrase from a number of your writings—all with full credit to you in the text itself in addition to in the bibliography
I have always been particularly impressed with the Introduction to your So-Called Alternative Medicine (SCAM) for Cancer as well as parts of your earlier book SCAM: So-Called Alternative Medicine (which carries my testimonial to you on the back cover!). This includes your definition of SCAM and your list of popular therapies and perhaps some of your common assumptions about SCAM. I also would love to draw upon some of the information in your boxes in the opening parts of SCAM…
Ken has on many occasions been most helpful and kind to me, and it goes without saying that I was delighted to assist.
He was a giant amongst the US skeptics, and we will all miss him badly.
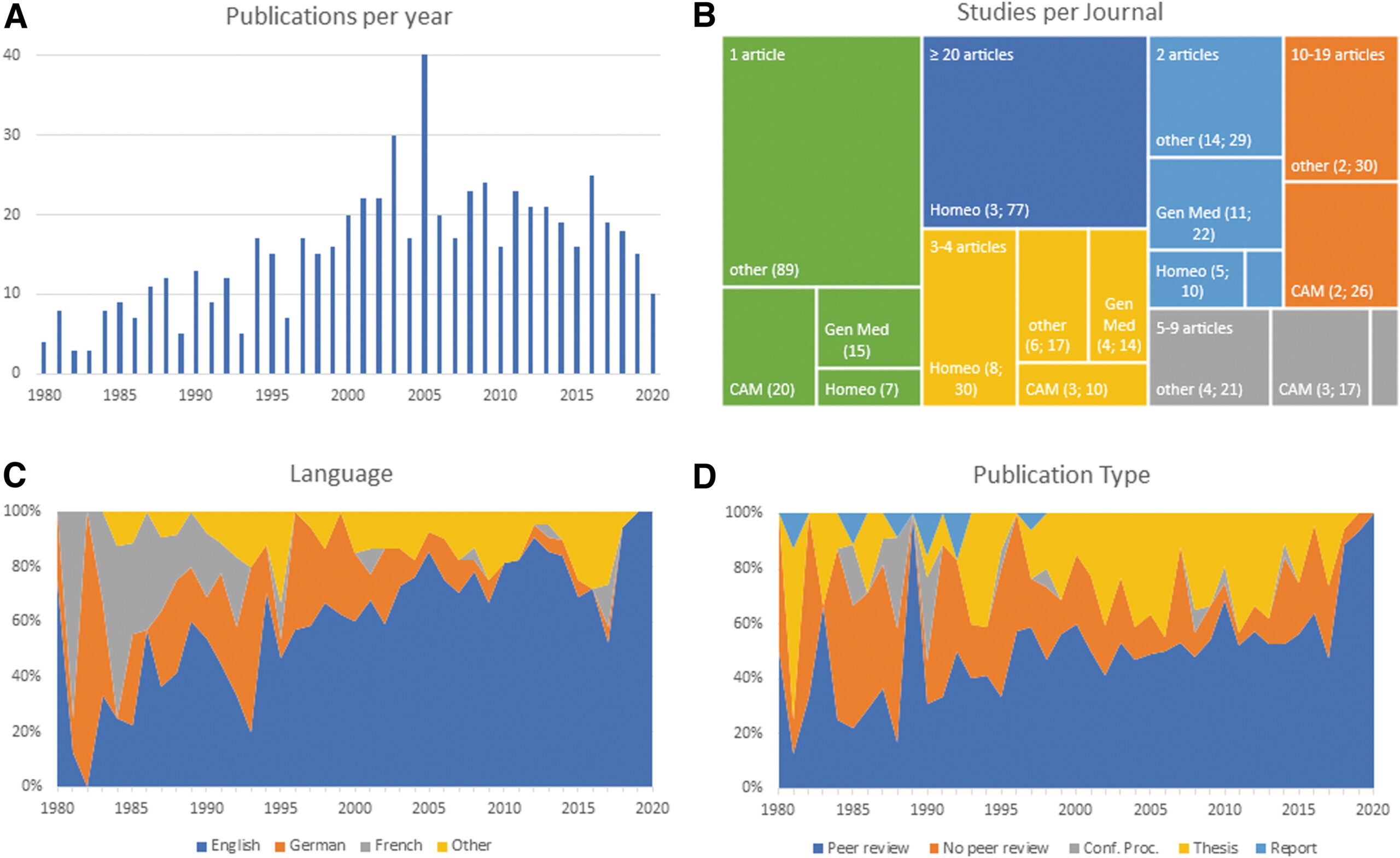
The authors of this article searched 37 online sources, as well as print libraries, for homeopathy (HOM) and related terms in eight languages (1980 to March 2021). They included studies that compared a homeopathic medicine or intervention with a control regarding the therapeutic or preventive outcome of a disease (classified according to International Classification of Diseases-10). Subsequently, the data were extracted independently by two reviewers and analyzed descriptively.
A total of 636 investigations met the inclusion criteria, of which 541 had a therapeutic and 95 a preventive purpose. Seventy-three percent were randomized controlled trials (n = 463), whereas the rest were non-randomized studies (n = 173). The most frequently employed comparator was placebo (n = 400).
The type of homeopathic intervention was classified as:
- multi-constituent or complex (n = 272),
- classical or individualized (n = 176),
- routine or clinical (n = 161),
- isopathic (n = 19),
- various (n = 8).
The potencies ranged from 1X (dilution of -10,000) to 10 M (100–10.000). The included studies explored the effect of HOM in 223 different medical indications. The authors also present the evidence in an online database.
The authors concluded that this bibliography maps the status quo of clinical research in HOM. The data will serve for future targeted reviews, which may focus on the most studied conditions and/or homeopathic medicines, clinical impact, and the risk of bias of the included studies.
There are still skeptics who claim that no evidence exists for homeopathy. This paper proves them wrong. The number of studies may seem sizable to homeopaths, but compared to most other so-called alternative medicines (SCAMs), it is low. And compared to any conventional field of healthcare, it is truly tiny.
There are also those who claim that no rigorous trials of homeopathy with a positive results have ever emerged. This assumption is also erroneous. There are several such studies, but this paper was not aimed at identifying them. Obviously, the more important question is this: what does the totality of the methodologically sound evidence show? It fails to convincingly demonstrate that homeopathy has effects beyond placebo.
The present review was unquestionably a lot of tedious work, but it does not address these latter questions. It was published by known believers in homeopathy and sponsored by the Tiedemann Foundation for Classical Homeopathy, the Homeopathy Foundation of the Association of Homeopathic Doctors (DZVhÄ), both in Germany, and the Foundation of Homeopathy Pierre Schmidt and the Förderverein komplementärmedizinische Forschung, both in Switzerland.
The dataset established by this article will now almost certainly be used for numerous further analyses. I hope that this work will not be left to enthusiasts of homeopathy who have often demonstrated to be blinded by their own biases and are thus no longer taken seriously outside the realm of homeopathy. It would be much more productive, I feel, if independent scientists could tackle this task.