commercial interests
In Germany, the anti-vax movement is frighteningly strong and it constitutes one of the main reasons for the relatively immunization rate. In no small part, this is due to the many anti-vax Heilpraktiker who practice in Germany. In an attempt to put the record straight, the ‘Verband Klassischer Homöopathen Deutschlands’ (VKHD, Association of Classical Homeopaths of Germany) recently published an article entitled ‘Heilpraktiker – Homeopathy – Vaccination’ (Heilpraktiker – Homöopathie – Impfen). Here is a short excerpt (my translation):
… There is a clear conceptual similarity between homeopathy and vaccination [1]. From a historical point of view, this was already reflected in the early days of homeopathy, when its discoverer, Samuel Hahnemann, expressed himself very positively with regard to the smallpox vaccination newly introduced at that time [2]. Thus, it is historically wrong to insinuate that users of homeopathy have a fundamentally negative attitude towards vaccinations [3]. In this context, terms such as “vaccination opponents” or “vaccination refusers” are misleading and defamatory [4].
A critical (not skeptical) approach to the topic of vaccinations is basically a characteristic of people with medical expertise. Such an attitude corresponds to the critical consideration necessary in daily practice and in each individual case to advise on suitable therapy options [5]. Properly working alternative practitioners give differentiated advice accordingly [6]. A fundamentally vaccine-rejecting attitude is precisely not a characteristic of a critical assessment that has taken place. The same applies to an unreflective recommendation of vaccinations or therapy methods, without taking into account individual factors as well as scientific and social backgrounds [7].
For the VKHD, we cannot give exact figures on recovered, vaccinated, or unvaccinated members. It is not the responsibility of a professional association to demand such information from its members [8]. We assume that alternative practitioners who provide information on vaccinations do so in accordance with a responsible ethical attitude, regardless of their own vaccination status [9] …
I have taken the liberty of inserting some references into this text. They relate to my comments, which are as follows:
- A conceptual similarity between vaccination and homeopathy exists only in the minds of homeopaths. They often claim that both use highly diluted remedies. This is wrong because homeopathic remedies do not usually contain active ingredients, whereas vaccines do. This fact also explains why homeopathics do not produce immune reactions, whereas vaccines do.
- Correct! Hahnemann was in favor of vaccination. That is why he would be ashamed today if he knew how many homeopaths oppose vaccination.
- What has this got to do with ‘historical’? I assume that the ‘insinuations’ refer to the situation today. Further, I don’t think anyone is suggesting that all homeopaths are ‘fundamentally’ opposed to vaccination. However, that many of them are anti-vaxers is an indisputable fact.
- I would rather think they are accurate.
- Correct.
- How can they without any medical background?
- Is it to be implied here that real medical people do?
- Maybe not ‘demand’, but inquire or request would be possible and desirable, wouldn’t it?
- It is nice that you believe this. But belief is not evidence.
Osteopathic manipulative treatment (OMT) is advocated not merely for spinal or musculoskeletal problems, as many consumers seem to think, osteopaths also claim it to be effective for (almost) every condition. Some osteopaths who believe in the gospel of Andrew Still, the founder of osteopathy, recommend it even to facilitate breastfeeding.
But is it effective?
A double-blind randomised controlled trial to answer this question was conducted between July 2013 and March 2016. Breastfed term infants were eligible if one of the following criteria was met: suboptimal breastfeeding behaviour, maternal cracked nipples or maternal pain. The infants were randomly assigned to the intervention or the control group. The intervention consisted of two sessions of early OMT, while in the control group, the manipulations were performed on a doll behind a screen. The primary outcome was the exclusive breastfeeding rate at 1 month, which was assessed in an intention-to-treat analysis. Randomisation was computer generated and only accessible to the osteopath practitioner. The parents, research assistants and paediatricians were masked to group assignment.
One hundred twenty-eight mother-infant dyads were randomised, with 64 assigned to each group. In each group, five infants were lost to follow-up. In the intervention group, 31 of 59 (53%) of infants were still exclusively breastfed at 1 month vs 39 of 59 (66%) in the control group, (OR 0.55, 95% CI 0.26 to 1.17; p=0.12). After adjustment for suboptimal breastfeeding behaviour, caesarean section, use of supplements and breast shields, the adjusted OR was 0.44 (95% CI 0.17 to 1.11; p=0.08). No adverse effects were reported in either group.
The authors concluded succinctly that OMT did not improve exclusive breastfeeding at 1 month.
Surprised?
Suppose not!
The only question that I can think of is this: why did osteopaths ever think that OMT might facilitate breastfeeding?
Protection against electromagnetic fields has been a topic before (see here and here). In so-called alternative medicine (SCAM) entrepreneurs have been quick to sell all sorts of ‘protective’ gadgets to the often all too gullible public. The devices are based on two main assumptions:
- EMF causes ill health.
- The device prevents this from happening.
Neither of them is correct, and the harm done by the claim is substantial. It can be measured in £ or $, because these gadgets are, of course, not cheap. Now a new type of harm is in the spotlight.
It has been reported that the Dutch authority for nuclear safety and radiation protection (ANVS) found several of these devices claimed to protect against 5G networks gave off harmful ionising radiation. It urged people not to use the products, which could cause harm in the long term.
The World Health Organization assures us that 5G mobile networks are safe, and not fundamentally different from existing 3G and 4G signals. They emit non-ionizing radio waves that do not damage DNA. But the marketers of these devices claim otherwise and many consumers believe them. This explains why there have been attacks on transmitters by people who believe 5G is harmful. The Guardian reported that, last year, 15 EU member states called on the European Commission to address a spate of conspiracy theories that had led to arson attacks against telecommunications masts.
The products identified included:
- “Energy Armor” sleeping mask,
- “Energy Armor” bracelet,
- “Energy Armor” necklace,
- Magnetix Wellness, a device for children.
Despite clear evidence that EMF protection is an expensive scam, Kim Jobst, Visiting Professor of Healthcare and Integrated Medicine Oxford Brooks University UK and former editor of the notorious JACM, stated the following about such a gadget: “Emerging evidence from early clinical, cellular and molecular studies of the effects of QLink on cardiovascular, immune and central nervous systems is startling.”
In May 2020, the UK’s Trading Standards sought to halt sales of a £339 USB stick that claimed to offer “protection” from 5G. “Anti-radiation stickers” have also been sold on Amazon. On this blog, we have discussed EMF devices that cost well over £4000.
This study assessed the effectiveness of Oscillococcinum in the protection from upper respiratory tract infections (URTIs) in patients with COPD who had been vaccinated against influenza infection over the 2018-2019 winter season.
A total of 106 patients were randomized into two groups:
- group V received influenza vaccination only
- group OV received influenza vaccination plus Oscillococcinum® (one oral dose per week from inclusion in the study until the end of follow-up, with a maximum of 6 months follow-up over the winter season).
The primary endpoint was the incidence rate of URTIs (number of URTIs/1000 patient-treatment exposure days) during follow-up compared between the two groups.
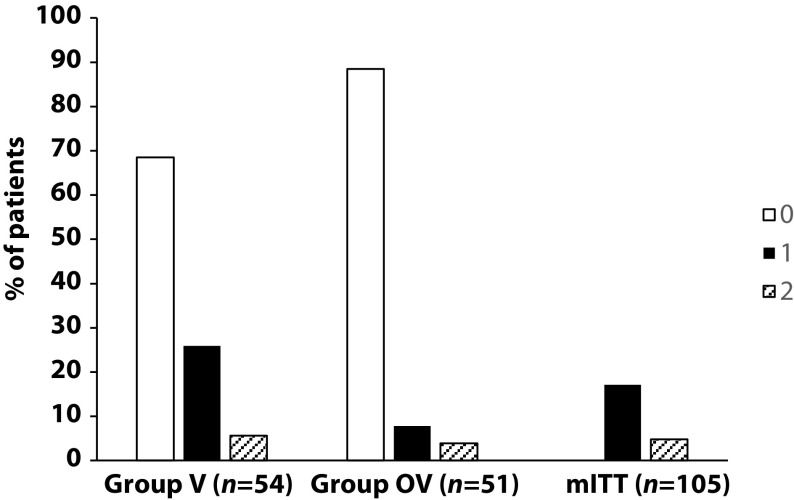
There was no significant difference in any of the demographic characteristics, baseline COPD, or clinical data between the two treatment groups (OV and V). The URTI incidence rate was significantly higher in group V than in group OV (2.9 versus 1.2 episodes/1000 treatment days, difference OV-V = -1.7; p=0.0312). There was a significant delay in occurrence of an URTI episode in the OV group versus the V group (mean ± standard error: 48.7 ± 3.0 versus 67.0 ± 2.8 days, respectively; p=0.0158). Limitations to this study include its small population size and the self-recording by patients of the number and duration of URTIs and exacerbations.
The authors concluded that the use of Oscillococcinum in patients with COPD led to a significant decrease in incidence and a delay in the appearance of URTI symptoms during the influenza-exposure period. The results of this study confirm the impact of this homeopathic medication on URTIs in patients with COPD.
Primary endpoint, comparison of the number of upper respiratory tract infections in the two treatment groups during follow-up
This prospective, randomized, single-center study was funded by Laboratoires Boiron, was conducted in the Pneumology Department of Charles Nicolle Hospital, Tunis, and was written up by a commercial firm specializing in writing for the pharmaceutical industry. The latter point may explain why it reads well and elegantly glosses over the many flaws of the trial.
If I did not know better, I might suspect that the study was designed to deceive us (Boiron would, of course, never do this!): The primary endpoint was the incidence rate of URTIs (number of URTIs/1000 patient-treatment exposure days) in the two groups during the follow-up period. This rate is calculated as the number of episodes of URTIs per 1000 days of follow-up/treatment exposure. The rates were then compared between the OV and V groups. The following symptoms were considered indicative of an URTI: fever, shivering, runny or blocked nose, sneezing, muscular aches/pain, sore throat, watery eyes, headaches, nausea/vomiting, diarrhoea, fatigue and loss of appetite.
This means that there was no verification whatsoever of the primary endpoint. In itself, this flaw would perhaps not be so bad. But put it together with the fact that patients were not blinded (there were no placebos!), it certainly is fatal.
In essence, the study shows that patients who perceive to receive treatment will also perceive to have fewer URTIs.
SURPRISE, SURPRISE!
Conversion therapy has been banned last week in Canada. These therapies – also known as sexual orientation change effort (SOCE), reparative therapy, reintegrative therapy, reorientation therapy, ex-gay therapy, and gay cure – rely on the assumption that sexual orientation can be changed, an idea long discredited by major medical associations in the US, the UK, France, and elsewhere. The new law makes “providing, promoting, or advertising conversion therapy” a criminal offense. It will also be an offense to profit from the provision of conversion therapy. In addition, the bill states a person cannot remove a “child from Canada with the intention that the child undergo conversion therapy outside Canada.” Prime Minister Justin Trudeau hailed the law’s Royal Assent: “It’s official: Our government’s legislation banning the despicable and degrading practice of conversion therapy has received Royal Assent — meaning it is now law.”
Conversion therapy is the attempt to change an individual’s sexual or gender identity by psychological, medical, or surgical interventions. Often, informed consent is insufficient or lacking. In conventional medicine, numerous treatments have been tried for this purpose, some of them dangerous and all of them ineffective. In alternative medicine, approaches that have been advocated include:
- Homeopathy (see below),
- Hypnotherapy,
- Spiritual healing,
- Prayer,
- Eye Movement Desensitization,
- Rebirthing,
- and others.
- Faith-based organizations or leaders
- Licensed healthcare professionals
- Unlicensed healthcare professionals
As previously reported, the German ‘Association of Catholic Doctors’ claimed that homeopathic remedies can cure homosexuality. Specifically, they advised that ‘…the working group ‘HOMEOPATHY’ of the Association notes homeopathic therapy options for homosexual tendencies…repertories contain special rubrics pointing to characteristic signs of homosexual behavior, including sexual peculiarities such as anal intercourse. And a homeopathic remedy called ‘Dr. Reckeweg R20 Glandular Drops for Women’ was claimed to treat “lesbian tendencies.” The product is “derived and potentised from fetal tissues.”
Several countries are now in the process of banning conversion therapy. France has already banned it and so has Germany. The UK government intends to introduce a legislative ban on the practice of conversion therapy. The consultation on how to best do this is open until 4 February 2022.
Compelling evidence has long shown that diagnostic imaging for low back pain does not improve care in the absence of suspicion of serious pathology. However, the effect of imaging use on clinical outcomes has not been investigated in patients presenting to chiropractors. The aim of this study was to determine if diagnostic imaging affects clinical outcomes in patients with low back pain presenting for chiropractic care.
A matched observational study using prospective longitudinal observational data with a one-year follow-up was performed in primary care chiropractic clinics in Denmark. Data were collected from November 2016 to December 2019. Participants included low back pain patients presenting for chiropractic care, who were either referred or not referred for diagnostic imaging at their initial visit. Patients were excluded if they were younger than 18 years, had a diagnosis of underlying pathology, or had previously had imaging relevant to their current clinical presentation. Coarsened exact matching was used to match participants referred for diagnostic imaging with participants not referred for diagnostic imaging on baseline variables including participant demographics, pain characteristics, and clinical history. Mixed linear and logistic regression models were used to assess the effect of imaging on back pain intensity and disability at two weeks, three months, and one year, and on global perceived effect and satisfaction with care at two weeks.
A total of 2162 patients were included, and 24.1% of them were referred for imaging. Near perfect balance between matched groups was achieved for baseline variables except for age and leg pain. Participants referred for imaging had slightly higher back pain intensity at two weeks (0.4, 95%CI: 0.1, 0.8) and one year (0.4, 95%CI: 0.0, 0.7), and disability at two weeks (5.7, 95%CI: 1.4, 10.0), but these differences are unlikely to be clinically meaningful. No difference between groups was found for the other outcome measures. Similar results were found when a sensitivity analysis, adjusted for age and leg pain intensity, was performed.
The authors concluded that diagnostic imaging did not result in better clinical outcomes in patients with low back pain presenting for chiropractic care. These results support that current guideline recommendations against routine imaging apply equally to chiropractic practice.
This study confirms what most experts suspected all along and what many chiropractors vehemently denied for years. One could still argue that the outcomes do not differ much and therefore imaging does not cause any harm. This argument would, however, be wrong. The harm it causes does not affect the immediate clinical outcomes. Needless imaging is costly and increases the cancer risk.
Yesterday, it was announced that the new German health secretary will be Dr. Karl Lauterbach. This seems a most reasonable choice (when did the UK last have a physician in that post?), and I certainly wish him the best of luck in his new position.
Lauterbach studied medicine at the RWTH Aachen University, University of Texas at San Antonio and University of Düsseldorf, where he graduated. From 1989 to 1992, he studied health policy and management as well as epidemiology at the Harvard School of Public Health in Boston, graduating with a Doctor of Science in 1992. From 1992 to 1993, he held a fellowship at the Harvard Medical School.
From 1998 until 2005, Lauterbach served as the director of the Institute of Health Economics and Clinical Epidemiology (IGKE) at the University of Cologne. He was appointed adjunct professor at the Harvard School of Public Health in 2008. He was a member of the Sachverständigenrat zur Begutachtung der Entwicklung im Gesundheitswesen (the council of experts advising the federal government on developments in the German healthcare system) from 1999 until he was elected to the Bundestag in September 2005.
But why does his appointment put the German defenders of homeopathy in a panic? The reason is simple: Lauterbach has in the past repeatedly argued against the reimbursement of homeopathy in Germany. This is, for instance, what DER SPIEGEL wrote in 2019 (my translation):
SPD parliamentary group vice-chairman Karl Lauterbach wants to prohibit public health insurance companies from reimbursing the costs of homeopathy. “We have to talk about this in the coalition,” he told the “Tagesspiegel”. Health insurance companies in Germany are not obliged to cover the costs of homeopathic treatments. However, they can pay for it voluntarily.
Voluntary benefits by health insurers must also be economically and medically reasonable, Lauterbach argues, referring to a similar push in France. According to the French Supreme Health Authority (HAS), the funds do not have sufficient scientific effect. The Ministry of Health had previously commissioned the HAS with the examination. It is considered likely that the French government will soon abolish the coverage of costs.
“In the spirit of reason and education as well as patient protection, it is also wrong in Germany for insurance companies to pay for homeopathy for marketing reasons,” Lauterbach wrote on Twitter in reaction to the decision in France. His demand is not new. Lauterbach had already spoken out in 2010 for a ban on the assumption of costs.
Many observers expect that Lauterbach – after getting the pandemic under control (not an easy task by any measure) – will indeed stop the reimbursement of homeopathy. Germany’s largest homeopathy producer reacted swiftly and is currently running an expensive campaign with full-page advertisements in German newspapers trying to improve the much-damaged public image of homeopathy:
In the advertisement above, for instance, it is implied that homeopaths are all in favor of vaccination. Regular readers of my blog will know that this is not true…
… and so does Dr. Lauterbach!
Dr. Mehmet Oz is one of the most influential promoters of outright quackery. I once (many years ago) met him at a meeting where we both were lecturing. My impression was that he does not believe a single word he speaks. Oz later became a TV star and had ample occasion to confirm my suspicion.
Oz’s wife, Lisa, is a Reiki master and has spoken widely of her insights into energy and health. Mehmet Oz appeared as a health expert on The Oprah Winfrey Show. In 2009, Winfrey offered to produce a syndicated series. The Dr. Oz Show debuted in September 2009 and became the most successful promotion of charlatanery in the US. During a Senate hearing on consumer protection in 2014, Senator Claire McCaskill stated that “the scientific community is almost monolithic against you” for airing segments on weight loss products that are later cited in advertisements, concluding that Oz plays a role, intentional or not, in perpetuating these scams, and that she is “concerned that you are melding medical advice, news, and entertainment in a way that harms consumers.” This judgement was supported by a 2014 analysis published in the BMJ; here is the abstract:
Objective To determine the quality of health recommendations and claims made on popular medical talk shows.
Design Prospective observational study.
Setting Mainstream television media.
Sources Internationally syndicated medical television talk shows that air daily (The Dr Oz Show and The Doctors).
Interventions Investigators randomly selected 40 episodes of each of The Dr Oz Show and The Doctors from early 2013 and identified and evaluated all recommendations made on each program. A group of experienced evidence reviewers independently searched for, and evaluated as a team, evidence to support 80 randomly selected recommendations from each show.
Main outcomes measures Percentage of recommendations that are supported by evidence as determined by a team of experienced evidence reviewers. Secondary outcomes included topics discussed, the number of recommendations made on the shows, and the types and details of recommendations that were made.
Results We could find at least a case study or better evidence to support 54% (95% confidence interval 47% to 62%) of the 160 recommendations (80 from each show). For recommendations in The Dr Oz Show, evidence supported 46%, contradicted 15%, and was not found for 39%. For recommendations in The Doctors, evidence supported 63%, contradicted 14%, and was not found for 24%. Believable or somewhat believable evidence supported 33% of the recommendations on The Dr Oz Show and 53% on The Doctors. On average, The Dr Oz Show had 12 recommendations per episode and The Doctors 11. The most common recommendation category on The Dr Oz Show was dietary advice (39%) and on The Doctors was to consult a healthcare provider (18%). A specific benefit was described for 43% and 41% of the recommendations made on the shows respectively. The magnitude of benefit was described for 17% of the recommendations on The Dr Oz Show and 11% on The Doctors. Disclosure of potential conflicts of interest accompanied 0.4% of recommendations.
Conclusions Recommendations made on medical talk shows often lack adequate information on specific benefits or the magnitude of the effects of these benefits. Approximately half of the recommendations have either no evidence or are contradicted by the best available evidence. Potential conflicts of interest are rarely addressed. The public should be skeptical about recommendations made on medical talk shows.
During the presidential campaign in 2016, Oz supported Trump and hosted him on his TV show. In 2018, Donald Trump appointed him to the President’s Council on Sports, Fitness, and Nutrition, Oz was criticized as an example of choosing “pundits over experts”. Recently, Oz announced he intends to run for the U.S. Senate as a Republican.
In April 2020, Oz also spurred controversy because he said that children should be sent back into schools despite the fact that the novel coronavirus pandemic had only just begun and there were no vaccines or therapeutics yet available. “I tell you, schools are a very appetizing opportunity,” he said, claiming that resuming classes “may only cost us 2 to 3 percent in terms of total mortality,” according to his “reading” of medical journals. The mistake was so substantial that Oz later provided a kind of half-apology, saying that he “misspoke.”
The effectiveness of manipulation versus mobilization for the management of spinal conditions, including cervicogenic headache, is conflicting, and a pragmatic approach comparing manipulation to mobilization has not been examined in a patient population with cervicogenic headache.
This study evaluated the effectiveness of manipulation compared to mobilization applied in a pragmatic fashion for patients with cervicogenic headache.
Forty-five (26 females) patients with cervicogenic headache were randomly assigned to receive either pragmatically selected manipulation or mobilization. Outcomes were measured at baseline, the second visit, discharge, and 1-month follow-up. The endpoints of the study included the Neck Disability Index (NDI), Numeric Pain Rating Scale (NPRS), the Headache Impact Test (HIT-6), the Global Rating of Change (GRC), the Patient Acceptable Symptoms Scale (PASS). The primary outcome measures were the effects of treatment on disability and pain. They were examined with a mixed-model analysis of variance (ANOVA), with treatment group (manipulation versus mobilization) as the between-subjects variable and time (baseline, 48 hours, discharge, and follow-up) as the within-subjects variable.
The interaction for the mixed model ANOVA was not statistically significant for NDI (p = 0.91), NPRS (p = 0.81), or HIT (p = 0.89). There was no significant difference between groups for the GRC or PASS.
The authors concluded that manipulation has similar effects on disability, pain, GRC, and cervical range of motion as mobilization when applied in a pragmatic fashion for patients with cervicogenic headaches.
Essentially, this study is an equivalence trial comparing one treatment to another. As such it would need a much larger sample size than the 45 patients enrolled by the investigators. If, however, we ignored this major flaw and assumed the results are valid, they would be consistent with both manipulation and mobilization being pure placebos.
I can imagine that many chiropractors find this conclusion unacceptable. Therefore, let me offer an alternative: both approaches were equally effective. Therefore, mobilization, which is associated with far fewer risks, is preferable. This means that patients suffering from cervicogenic headache should see an osteopath who is less likely to use manipulation than a chiropractor.
And again, I can imagine that many chiropractors find this conclusion unacceptable.
The complex links between so-called alternative medicine (SCAM) and the pandemic have been a regular subject on this blog. Here is more:
This study investigated if people’s response to the official recommendations during the COVID-19 pandemic is associated with conspiracy beliefs related to COVID-19, a distrust in the sources providing information on COVID-19, and an endorsement of SCAM.
The sample consisted of 1325 Finnish adults who filled out an online survey advertised on Facebook. Structural regression analysis was used to investigate whether:
1) conspiracy beliefs, a distrust in information sources, and endorsement of SCAM predict people’s response to the non-pharmaceutical interventions (NPIs) implemented by the government during the COVID-19 pandemic,
2) conspiracy beliefs, a distrust in information sources, and endorsement of CAM are related to people’s willingness to take a COVID-19 vaccine.
The results indicate that individuals with more conspiracy beliefs and lower trust in information sources were less likely to have a positive response to the NPIs. Individuals with less trust in information sources and more endorsement of SCAM were more unwilling to take a COVID-19 vaccine. Distrust in information sources was the strongest and most consistent predictor in all models. In addition, the analyses revealed that some of the people who respond negatively to the NPIs also have a lower likelihood to take the vaccine. This association was partly related to lower trust in information sources.
The authors concluded that distrusting the establishment to provide accurate information, believing in conspiracy theories, and endorsing treatments and substances that are not part of conventional medicine, are all associated with a more negative response to the official guidelines during COVID-19. How people respond to the guidelines, however, is more strongly and consistently related to the degree of trust they feel in the information sources than to their tendency to hold conspiracy beliefs or endorse CAM. These findings highlight the need for governments and health authorities to create communication strategies that build public trust.
I also believe that these findings highlight the urgent need for improvements in education. In my view, it should start at school and continue into adult life. It should focus on a better understanding of science and – crucially – on the ability to differentiate facts from fiction and conspiracies.