commercial interests
An interesting and fully referenced (205 references) article caught my attention; it seems highly relevant to the discussions we are having on this blog. Let me show you the abstract:
Medical misinformation has always existed, but it has recently become more frequent due to the development of the internet and social media. Medical misinformation can cover a wide variety of topics, and studies show that some groups are more likely to be affected by medical misinformation than others, like those with less trust in health care, less health literacy, and a more positive attitude toward alternative medicines. Aspects of the internet, like echo chambers and algorithms, have contributed to the rise of medical misinformation, along with belief in anecdotal evidence and alternative remedies that are not backed by science. Some personal beliefs and a lack of media literacy skills are also contributing to medical misinformation. Medical misinformation causes higher rates of death and negative health outcomes, a lack of trust in medical professionals, and more racism and hate crimes. One possible way to combat the spread of misinformation is education surrounding media literacy. Still, there are gaps in this practice that must be addressed like a lack of high-quality research about different educational programs.
The author also offers the following key points:
- Medical misinformation is becoming an urgent issue for United States citizens—leading to increased deaths,
a lack of trust in health professionals, and hate crimes and racism. - Although this is a worldwide issue, the United States has the second highest rate of misinformation of any
country, behind India. - One piece of misinformation during the COVID-19 pandemic stated that highly concentrated alcohol could
disinfect the body and kill the virus. Studies show that 800 people died, 5,876 were hospitalized, and 60
became completely blind from drinking methanol, thinking it would cure coronavirus. - Studies estimate that only 14% of the United States population has proficient health literacy, which makes it difficult to recognize medical misinformation.
- Media literacy education is being pursued in order to combat the spread of misinformation, but more research is needed in order to understand the long-term effects of this education and what programs are best.
__________________
I would like to stress, as indeeed the author does as well, that medical misinformation is a phenomenon that is by no means confined to the US. Like most information, misinformation has become a global issue. Its dangers cannot be under-estimated. My blog offers an abundance of reports where misinformation in the realm of so-called alternative medicine (SCAM) has caused harm and even death. The author advocates media literacy as a remedy for the problem. I would argue that even more important would be to teach CRITICAL THINKING, a task that has to start at school and must continue well into adult life.
This conclusion is so very obvious that it begs an important question: WHY HAS IT NOT BEEN DONE YEARS AGO? The answer, I fear, is simple: for reasons that are self-evident, governments have little interst in the public being able to think critically. On the contrary, governments across the world foremost want to be re-elected, and critical thinking would be a major obstacle to this aim.
It has been reported that 5 people who took a Japanese health supplement have died and more than 100 have been hospitalized as of Friday, a week after a pharmaceutical company issued a recall of the products, officials said. Osaka-based Kobayashi Pharmaceutical Co. came under fire for not going public quickly with problems known internally as early as January. Yet the first public announcement came only on 22 March. Company officials said 114 people were being treated in hospitals after taking products — including Benikoji Choleste Help meant to lower cholesterol — that contain an ingredient called benikoji, a red species of mold. Some people developed kidney problems after taking the supplements, but the exact cause was still under investigation in cooperation with government laboratories, according to the manufacturer.
“We apologize deeply,” President Akihiro Kobayashi told reporters last Friday, bowing for a long time to emphasize the apology alongside three other top company officials. He expressed remorse to those who have died and have been taken ill and to their families. He also apologized for the troubles caused to the entire health food industry and the medical profession, adding that the company was working to prevent further damage and improve crisis management.
The company’s products have been recalled — as have dozens of other products that contain benikoji, including miso paste, crackers, and a vinegar dressing. Japan’s health ministry put up a list on its official site of all the recalled products, including some that use benikoji for food coloring. The ministry warned the deaths could keep growing. The supplements could be bought at drug stores without a prescription from a doctor, and some may have been purchased or exported before the recall, including by tourists who may not be aware of the health risks.
Kobayashi Pharmaceutical had been selling benikoji products for years, with a million packages sold over the past 3 fiscal years, but a problem crept up with the supplements produced in 2023. Kobayashi Pharmaceutical said it produced 18.5 tons of benikoji last year. Some analysts blame the recent deregulation initiatives, which simplified and sped up approval for health products to spur economic growth.
________________________
Anouther source reported that Japanese authorities on Saturday raided a drug factory after a pharmaceutical company reported at least five deaths and 114 hospitalizations possibly linked to a health supplement. About a dozen Japanese health officials walked into the Osaka plant of the Kobayashi Pharmaceutical Co., as seen in footage of the raid widely telecasted on Japanese news. The health supplement in question is a pink pill called Benikoji Choleste Help. It is said to help lower cholesterol levels. A key ingredient is benikoji, a type of red mold. The company has said it knows little about the cause of the sickness, which can include kidney failure. It is currently investigating the effects in cooperation with Japan’s government.
___________________________
More recent reports update the figure of affected individuals: Japanese dietary supplements at the center of an expanding health scare have now been linked to at least 157 hospitalizations, a health ministry official said Tuesday.The figure reflects an increase from the 114 hospitalization cases that Kobayashi Pharmaceutical said on Friday were linked to its products containing red yeast rice, or beni kōji.
A Kobayashi Pharmaceutical spokeswoman confirmed the latest hospitalization cases without elaborating further.
Benikoji is widely sold and used; not just in Japan. It comes under a range of different names:
- red yeast rice,
- red fermented rice,
- red kojic rice,
- red koji rice,
- anka,
- angkak,
- Ben Cao Gang Mu.
It is a bright reddish purple fermented rice which acquires its color from being cultivated with the mold Monascus purpureus. Red yeast rice is used as food and as a medicine in Asian cultures, such as Kampo and TCM.
It contains lovastatin which, of course, became patented and is marketed as the prescription drug, Mevacor. Red yeast rice went on to become a non-prescription dietary supplement in the United States and other countries. In 1998, the U.S. FDA banned a dietary supplement containing red yeast rice extract, stating that red yeast rice products containing monacolin K are identical to a prescription drug, and thus subject to regulation as a drug.
Whenever a journalist wants to discuss the subject of acupuncture with me, he or she will inevitably ask one question:
DOES ACUPUNCTURE WORK?
It seems a legitimate, obvious and simple question, particularly during ‘Acupuncture Awareness Week‘, and I have heard it hundreds of times. Why then do I hesitate to answer it?
Journalists – like most of us – would like a straight answer, like YES or NO. But straight answers are in short supply, particularly when we are talking about acupuncture.
Let me explain.
Acupuncture is part of ‘Traditional Chinese Medicine’ (TCM). It is said to re-balance the life forces that determine our health. As such it is seen as a panacea, a treatment for all ills. Therefore, the question, does it work?, ought to be more specific: does it work for pain, obesity, fatigue, hair-loss, addiction, anxiety, ADHA, depression, asthma, old age, etc.etc. As we are dealing with virtually thousands of ills, the question, does it work?, quickly explodes into thousands of more specific questions.
But that’s not all!
The question, does acupuncture work?, assumes that we are talking about one therapy. Yet, there are dozens of different acupuncture traditions and sites:
- body acupuncture,
- ear acupuncture,
- tongue acupuncture,
- scalp acupuncture,
- etc., etc.
Then there are dozens of different ways to stimulate acupuncture points:
- needle acupuncture,
- electroacupuncture,
- acupressure,
- moxibustion,
- ultrasound acupuncture,
- laser acupuncture,
- etc., etc.
And then there are, of course, different acupuncture ‘philosophies’ or cultures:
- TCM,
- ‘Western’ acupuncture,
- Korean acupuncture,
- Japanese acupuncture,
- etc., etc.
If we multiply these different options, we surely arrive at thousands of different variations of acupuncture being used for thousands of different conditions.
But this is still not all!
To answer the question, does it work?, we today have easily around 10 000 clinical trials. One might therefore think that, despite the mentioned complexity, we might find several conclusive answers for the more specific questions. But there are very significant obstacles that are in our way:
- most acupuncture trials are of lousy quality;
- most were conducted by lousy researchers who merely aim at showing that acupuncture works rather that testing whether it is effective;
- most originate from China and are published in Chinese which means that most of us cannot access them;
- they get nevertheless included in many of the systematic reviews that are currently being published without non-Chinese speakers ever being able to scrutinise them;
- TCM is a hugely important export article for China which means that political influence is abundant;
- several investigators have noted that virtually 100% of Chinese acupuncture trials report positive results regardless of the condition that is being targeted;
- it has been reported that about 80% of studies emerging from China are fabricated.
Now, I think you understand why I hesitate every time a journalist asks me:
DOES ACUPUNCTURE WORK?
Most journalists do not have the patience to listen to all the complexity this question evokes. Many do not have the intellectual capacity to comprehend an exhaustive reply. But all want to hear a simple and conclusive answer.
So, what do I say in this situation?
Usually, I respond that the answer would depend on who one asks. An acupuncturist is likely to say: YES, OF COURSE, IT DOES! An less biased expert might reply:
IT’S COMPLEX, BUT THE MOST RELIABLE EVIDENCE IS FAR FROM CONVINCING.
The aim of this study was to establish an international consensus regarding the use of spinal manipulation and mobilisation among infants, children, and adolescents among expert international physiotherapists. Twenty-six international expert physiotherapists in manual therapy and paediatrics voluntarily participated in a 3-Round Delphi survey to reach a consensus via direct electronic mail solicitation using Qualtrics®. Consensus was defined a-priori as ≥75% agreement on all items with the same ranking of agreement or disagreement. Round 1 identified impairments and conditions where spinal mobilisation and manipulation might be utilised. In Rounds 2 and 3, panelists agreed or disagreed using a 4-point Likert scale.
Eleven physiotherapists from seven countries representing five continents completed all three Delphi rounds. Consensus regarding spinal mobilisation or manipulation included:
● Manipulation is not recommended: (1) for infants across all conditions, impairments, and
spinal levels; and (2) for children and adolescents across most conditions and spinal levels.
● Manipulation may be recommended for adolescents to treat spinal region-specific joint
hypomobility (thoracic, lumbar), and pain (thoracic).
● Mobilisation may be recommended for children and adolescents with hypomobility, joint
pain, muscle/myofascial pain, or stiffness at all spinal levels.
The authors of this paper concluded that consensus revealed spinal manipulation should not be performed on infants regardless of condition, impairment, or spinal level. Additionally, the panel agreed that manipulation may be recommended only for adolescents to treat joint pain and joint hypomobility (limited to thoracic and/or lumbar levels). Spinal mobilisation may be recommended for joint hypomobility, joint pain, muscle/myofascial pain, and muscle/myofascial stiffness at all spinal levels among children and adolescents.
Various forms of spinal manipulations are the hallmark therapy of chiropractors. Almost 100% of their patients recieve these interventions. So, what will our friends, the chiros, say about the consensus? Might it be this:
- Physiotherapists are not the experts on spinal manipulation.
- Only chiropractors can do them properly.
- And when WE do them, they are very good*!
(* for our income)
This study aims to assess the feasibility of a pragmatic prospective study aiming to report the immediate and delayed (48-hours post-treatment) AEs associated with manual therapies in children aged 5 or younger and to report preliminary data on AEs frequency.
Between July 2021 and March 2022, chiropractors were recruited through purposive sampling and via a dedicated Facebook group for Quebec chiropractors interested in pediatrics. Legal guardians of patients aged 5 or younger were invited to fill out an online information and consent form. AEs were collected using the SafetyNET reporting system, which had been previously translated by the research team. Immediate AEs were collected through a questionnaire filled out by the legal guardian immediately after the treatment, while delayed AEs were collected through a questionnaire sent by email to the legal guardian 48 h after the treatment. Feasibility was assessed qualitatively through feedback from chiropractors and quantitatively through recruitment data.
Overall, a total of 28 chiropractors expressed interest following the Facebook publication, and 5 participated. An additional two chiropractors were enrolled through purposive sampling. In total, 80 legal guardians consented to their child’s participation, and data from 73 children were included for the analysis of AEs. At least one AE was reported in 30% of children (22/73), and AEs were mainly observed immediately following the treatment (16/22). The most common AEs were irritability/crying (11 children) or fatigue/tiredness (11 children). Feasibility analysis demonstrated that regular communication between the research team and clinicians, as well as targeting clinicians who showed great interest in pediatrics, were key factors for successful research.
The authors concluded that their results suggest that it is feasible to conduct a prospective pragmatic study evaluating AEs associated with manual therapies in private practices. Direct communication with the clinicians, a strategic clinicians’ recruitment plan, and the resulting administrative burden should be considered in future studies. A larger study is required to confirm the frequency of AEs reported in the current study.
It is hardly surprising that such a study is ‘feasible’. I could have told the authors that and saved them the trouble of doing the study. What is surprising, in my view, that chiropractors, after ~120 years of existence of the profession, ask whether it is feasible.
I suggest to do the definitive study on a much larger sample, extend the observation period, and recruit a representative rather than self-selected sample of chiros … or – much better – forget about the study and establich a functioning post-marketing surveillance system.
An article about chiropractic caught my attention. Let me show you its final section which, I think, is relevant to what we often discuss on this blog:
If chiropractic treatment is unscientific, then why do I feel better? Because lots of things alleviate pain. Massage, analgesia and heat – but also a provider who listens, empathises and bothers to examine a patient. Then there is the placebo effect. For centuries, doctors have recognised that different interventions with unclear pathways result in clinical improvement. Among the benefits patients attributed to placebo 100 years ago: “I sleep better; my appetite is improved; my breathing is better; I can walk further without pain in my chest; my nerves are steadier.” Nothing has changed. Pain is a universal assignment; no one has a monopoly on its relief.
The chiropractic industry owes its existence to a ghost. Its founder, David Palmer, wrote in his memoir The Chiropractor that the principles of spinal manipulation were passed on to him during a séance by a doctor who had been dead for half a century. Before this, Palmer was a “magnetic healer”.
Today, chiropractors preside over a multibillion-dollar regulated industry that draws patients for various reasons. Some can’t find or afford a doctor, feel dismissed, or worse, mistreated. Others mistrust the medical establishment and big pharma. Still others want natural healing. But none of these reasons justifies conflating a chiropractor with a doctor. The conflation feels especially hazardous in an environment of health illiteracy, where the mere title of doctor confers upon its bearer strong legitimacy.
Chiropractors don’t have the same training as doctors. They cannot issue prescriptions or order advanced imaging. They do not undergo lifelong peer review or open themselves to monthly morbidity audits.
I know that doctors could do with a dose of humility, but I can’t find any evidence (or the need) for the assertion on one website that chiropractors are “academic overachievers”. Or the ambit claim that most health professionals have no idea how complicated the brain is, but chiropractors do.
Forget doctors, patients deserve more respect.
My friend’s back feels better for now. When it flares, I wonder if she will seek my advice – and I am prepared to hear no. Everyone is entitled to see a chiropractor. But no patient should visit a chiropractor thinking that they are seeing a doctor.
______________________
I would put it more bluntly:
- chiropractors are poorly trained; in particular, they do not learn to question their own, often ridiculous beliefs;
- they are poorly regulated; in the UK, the GCC seems to protect the chiros rather than the public;
- chiropractors regularly disregard essential rules of medical ethics, e.g. informed consent;
- many try to mislead us by pretending they are physicians;
- their hallmark intervention, spinal manipulation, can cause considerable harm;
- it generates hardly any demonstrable benefit for any condition;
- chiropractors also cause considerable harm, e.g. by interfering with real medicine, e.g. vaccinations;
- thus, in general, chiropractors do more harm than good;
- yes, everyone is entitled to see a chiropractor, but before they do, reliable information should be mandatory.
A recent article about ayurvedic medicine caught my eye. Here are a few excerpts:
Imagine if there were a magic pill to ward off COVID-19. Or if you could cure diabetes with vegetable juices and herbal pills instead of controlling it with insulin medication. Or if yoga and breathing exercises were all you need to do to get rid of asthma. These are all claims made by Patanjali Ayurved, one of India’s biggest manufacturers of traditional ayurvedic products…
Many scientists have expressed concerns over the lack of research into the safety and efficacy of ayurvedic products… Nonetheless, Ayurveda enjoys widespread acceptance among Indians. And under India’s Hindu-nationalist government that took power in 2014, ayurveda and other alternative systems of medicine have received unprecedented government support. India’s ministry of alternative medicine gets nearly $500 million a year. The government also promotes ayurveda through its international trade and diplomatic channels. All this set Patanjali’s fortunes soaring.
But now the Supreme Court of India has temporarily banned Patanjali – named after a Hindu mystic best known for his writings on yoga – from advertising some of its products… “The entire country has been taken for a ride,” Ahsanuddin Amanullah, one of the two judges conducting the court hearing, told the lawyer representing the government… The Indian Medical Association had brought the case to court in August 2022, claiming that Patanjali and its brand ambassador Baba Ramdev made a series of false claims against evidence-backed modern medicine and its practitioners, and spread misinformation about COVID-19 vaccines. Their petition also referred to instances where Ramdev lambasted modern medicine as a “stupid and bankrupt science” at a yoga session. The trigger was a series of Patanjali advertisements in Indian newspapers in July 2022 claiming that ayurvedic products could cure chronic conditions like diabetes, high blood pressure, heart diseases and autoimmune conditions. The Indian Medical Association’s petition alleged that such claims were in violation of India’s Drugs and Magic Remedies (Objectionable Advertisements) Act.
…The company’s public face – yoga guru Baba Ramdev – is a vocal supporter of India’s ruling party, the BJP, and Prime Minister Narendra Modi. Modi even inaugurated Patanjali’s ayurvedic research facility in 2017… Some scientists have accused their government of promoting these alternative medicines at the expense of modern medicine, partly as a way to glorify India’s culture and history. “One of the political ideas of this government is to glorify the Hindu tradition,” says Dhrubajyoti Mukherjee, president of the Breakthrough Science Society, an organization that promotes scientific thinking. “But in the name of our glorious past, the government is propagating obscure, unscientific ideas.”
… A few months after the outbreak of the COVID-19 pandemic in 2020, India’s health minister at the time, Harsh Vardhan participated in the company’s launch of pills, where Ramdev, the yoga guru, claimed the pills showed “100 percent favorable results” during clinical trials on patients. Despite experts flagging the lack of evidence, the company said it sold 2.5 million kits in six months, consisting of the tablets to ward off COVID-19 and bottled oils that would allegedly boost immunity. And the company is making an enormous amount of money: Its income was over $1.3 billion in the financial year 2021-22, with profits of $74 million before taxes.
Addressing the overall impact of misinformation about ayurvedic treatments, Dr. Jayesh Lele, vice president of the Indian Medical Association, says “Our worry is people are being misguided. We have got people who’ve left our treatment saying their kidneys will be able to function properly [using ayurvedic medicines] and ended up with renal failure. The same happened with patients suffering from hepatitis, who’ve got the wrong medicine and ended up with further problems. And if you say every day that modern medicine is bad, that is not acceptable.”
_______________
The sad thing, in my view, is that (as discussed previously) ayurvedic medicine has not just taken India for a ride:
- King Charles’ “Ayurvedic Centre of Excellence” turns out to be an embarrassing failure
- Dr Michael Dixon seems to support homeopathy as a treatment for cancer
- PRINCE CHARLES: the ‘immense value’ of alternative diagnostic methods
- Will the UK ‘ROYAL COLLEGE OF GENERAL PRACTITIONERS’ soon become a ‘ROYAL COLLEGE OF QUACKERY’?
And perhaps even more disappointing is the notion that, while in India they take action in order to prevent harm, I can see no such developments in the UK.
The Amercian Medical Association (AMA) recently published a lengthy article on naturopathy in the US. Here are some excerpts:
There are three types of health professionals who offer naturopathic treatment:
- Naturopathic doctors. These nonphysicians graduate from a four-year, professional-level program at an accredited naturopathic medical school, earning either the doctor of naturopathy (ND) degree or the doctor of naturopathic medicine (NMD) degree.
- Traditional naturopaths, who have obtained education through some combination of a mentorship program with another professional or at an alternative clinic, distance-learning program or classroom schooling on natural health, or other holistic studies.
- Other health professionals such as chiropractors, massage therapists, dentists, nurses, nutritionists, or physicians who practice under a professional license but include some naturopathic methods in their practice and who may have studied on their own or taken courses on naturopathic methods.
At least 24 states and the District of Columbia regulate the practice of naturopathy. In order to be licensed, naturopaths in these states must earn an ND or NMD from an accredited naturopathic program and pass the Naturopathic Physicians Licensing Exam. Three states—Florida, South Carolina and Tennessee—prohibit the practice of naturopathy. In states that neither license nor prohibit the practice of naturopathy, traditional naturopaths and NDs alike may practice without being subject to state regulation.
Postgraduate training is neither common nor required of graduates of naturopathic schools, except in Utah … less than 10% of naturopaths participate in an approved residency, and such residencies last only a year and lack a high degree of standardization.
… naturopaths are required to get at least 1,200 hours of direct patient contact, physicians get 12,000–16,000 hours of clinical training…
ND programs emphasize naturopathic principes—for example, the healing power of nature—and naturopathic therapeutics such as botanical medicine, homeopoathy and hydrotherapy. Coursework in naturopathic therapeutics is combined with, and taught alongside, coursework in sciences. But there are no specifications around the number of hours required in each area … naturopathic students may lack exposure to key clinical scenarios in the course of their training … naturopathic students’ clinical experience is typically gained through outpatient health care clinics, as naturopathic medical schools typically do not have significant hospital affiliation. This means there is no guarantee that a naturopathic student completing a clinical rotation will see patients who are actually sick or hospitalized, and they may not be exposed to infants, children, adolescents or the elderly. It has been said that naturopaths tend to treat the “worried well.”
… Naturopaths claim they are trained as primary care providers and, as such, are educated and trained to diagnose, manage and treat many conditions, including bloodstream infections, heart disease and autoimmune disorders. Yet their education and training falls several years and thousands of hours short of what physicians get.
…The AMA believes it is the responsibility of policymakers to ensure that naturopaths’ claims that they can treat a broad range of conditions are backed by facts—facts that include the specific education and training necessary to ensure patient safety.
________________
The AMA is clearly cautious here. A less polite statement might simply stress that naturopaths are taught a lot of nonsense which they later tend to administer to their unsuspecting patients. On this blog, we have repeatedly discussed the danger naturopaths present to public health in the US and elsewhere, e.g.:
- How reliable are the claims made by naturopathic influencers?
- Naturopath jailed for selling fraudulent vaccination documents
- Naturopath fined for misdiagnosing and treating a rectal tumor for hemorrhoids
- Naturopaths are ‘not bound by science,’ lawyer argues
- Vaccination rates of Canadian healthcare professionals: those of chiropractors and naturopaths are at the lowest
- Is veterinary naturopathy animal abuse?
- Naturopathic ‘cancer specialist’ using coffee enemas found guilty
- Patients consulting chiropractors, homeopaths, or naturopaths are less likely to agree to the flu jab
- A naturopath responsible for the death of two cancer patients was sentenced to two years
- A naturopath in court after two of his cancer patients died
- Many naturopaths, homeopaths, and chiropractors are a risk to public health
- Naturopath treats autism with fecal transplants
- A naturopath promoting fake news about COVID vaccinations
- Naturopathy (according to the WNF) = quackery steeped in obsolete fantasies
- Canadian naturopaths may no longer call themselves ‘medically trained’
- Naturopaths’ counselling against vaccinations could be criminally negligent
- Naturopathy for cancer … claims that have the potential to be lethal
- Severe liver injury due to naturopaths’ prescription of Epsom salt
- Naturopaths should not treat children
- Some naturopaths are clearly a danger to public health
- Death of a child through naturopathy
Claims that naturopaths are a viable alternative to evidence-based medicine are wrong, irresponsible and dangerous. Regulators must be reminded that they have the duty to protect the public from charlatans and should therefore ensure that no false therapeutic or diagnostic claims can be made by naturopaths.
Looking at some ancient papers of mine, I came across a short BMJ paper from 1994. Here is a passage from it:
… A standard letter (on departmental letterhead) was written (in German) to all 189 firms that we identified as marketing herbal drugs in Germany. It asked (among other questions) for reprints of articles reporting controlled clinical trials on the company’s product(s).
Only 19 replies had reached us six weeks later. Four of these included at least one reprint. Twelve respondents regretted not knowing of clinical trials on their drug(s). In three cases we had written to a wrong address (one
instance) or to a firm which did not market phytomedicines (two instances).
These data, though far from conclusive, do not give the impression that research is in proportion to either prevalence or financial tumover of herbal remedies…
I wonder what the results would be, if we repeated this little excercise today, 30 years afteer the original investigation. I fear that the findings would be much the same or perhaps even worse. I also suspect that they would be similar regardless of the country we chose. Those who sell herbal remedies have very little incentive to do expensive clinical trials to test whether the products they earn their money with actually work. They may be doing well without it and ask themselves, why spend money on research that might not show what we hope and could easily turn out to jeopardize our financial success?
But the problem is by no means confined to herbal manufacturers (who would arguably have an important share to initiate and sponsor research). Even though fundamental questions remain unanswered, research into herbal medicine is scarce across the board.
To see whether this statement is true, I did a very quick Medline search. It showed that, in 2023, just over 13 000 papers on herbal medicine emerged. Of those, just 460 were listed as clinical trials. The latter figure is almost certainly considerably smaller than the true amount because Medline is over-generous in classifying papers as clinical trials. I thus estimate that only around 200 clinical trials of herbal medicine are conducted each year. Considering that we are dealing with thousands of herbs and ten thousands of herbal products, this figure is an embarrassment for the sector – which, as we have seen just days ago, is doing extremely well in finacial terms.
I usually take ‘market reports’ with a pinch of salt. Having said that, this document makes some rather interesting predictions:
The size of the market for so-called alternative medicine (SCAM) is projected to expand from USD 147.7 billion in 2023 to approximately USD 1489.4 billion by the year 2033. This projection indicates a remarkable Compound Annual Growth Rate (CAGR) of 26% over the forecast period.
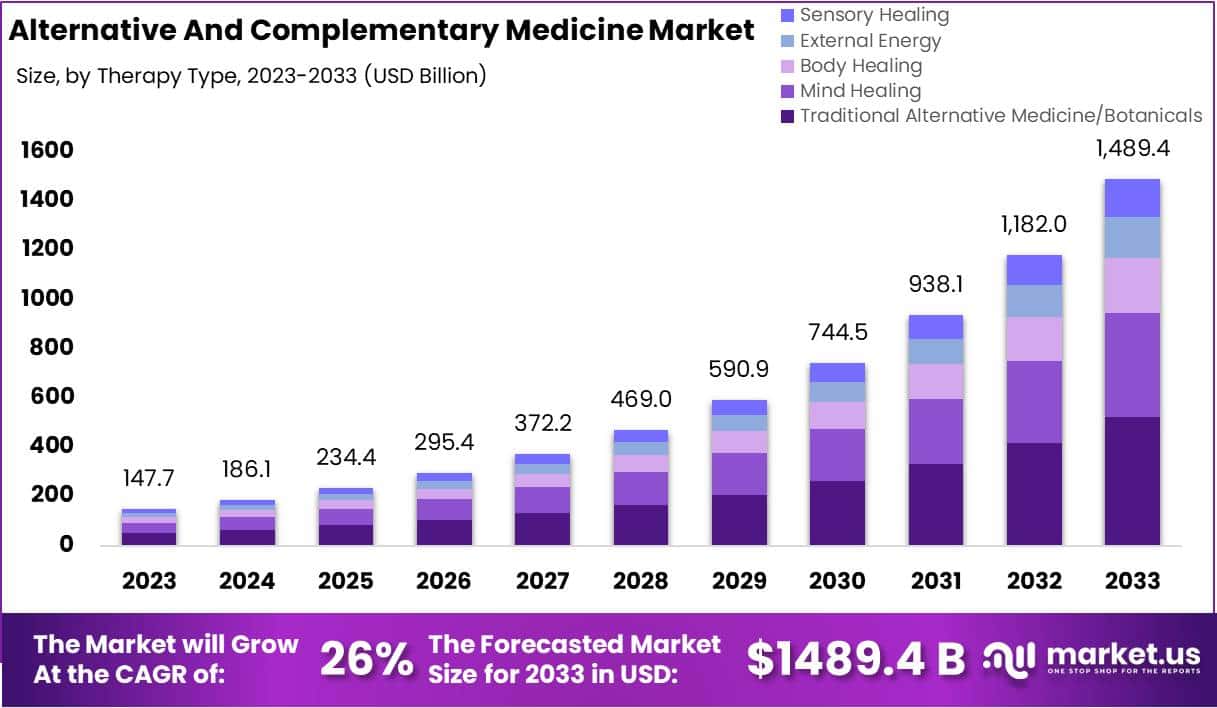
The market for SCAM is experiencing significant growth, fueled by increasing consumer interest in natural and holistic health solutions. This trend reflects a broader shift in societal attitudes towards health and wellness, emphasizing preventive care and natural health practices.
The market’s dynamics are influenced by various factors, including consumer preferences, regulatory standards, and evolving perceptions of health and wellness. As the popularity of these alternative therapies grows, it is crucial for individuals to consult with healthcare professionals to ensure that these non-conventional approaches are safely and effectively incorporated into their overall health regimen. The increasing acceptance of SCAM underscores a collective move towards more personalized and holistic healthcare solutions, resonating with today’s health-conscious consumers.
In 2023, Traditional Alternative Medicine/Botanicals led the market, capturing a 35.2% share, which reflects a strong consumer inclination towards these treatments. Dietary Supplements were prominent in the market, securing a 25.1% share in 2023, which underscores the high consumer demand for nutritional aids. Direct Sales were the most favored distribution channel, accounting for 43.2% of the market share in 2023, which indicates their significant impact on guiding consumer purchases. Pain Management was the predominant application area, holding a 24.9% market share in 2023, propelled by the growing acknowledgment of non-pharmacological treatment options. Adults represented a substantial portion of the market, making up 62.33% in 2023, signifying a marked preference for SCAM therapies within this age group. Europe stood out as the market leader, claiming a 42.6% share in 2023, a position supported by widespread acceptance, governmental backing, and an increasing elderly population. The regions of North America and Asia-Pacific are highlighted as areas with potential, signaling opportunities for market expansion beyond the European stronghold in the upcoming years.
Leading Market Players Are:
- Columbia Nutritional
- Nordic Nutraceuticals
- Ramamani Iyengar Memorial Yoga Institute
- The Healing Company Ltd.
- John Schumacher Unity Woods Yoga Centre
- Sheng Chang Pharmaceutical Company
- Pure encapsulations LLC.
- Herb Pharm
- AYUSH Ayurvedic Pte Ltd.
Recent developments:
- In December 2023, Adoratherapy launched the Alkemie Chakra Healing Line, an aromatherapy range aimed at harmonizing the seven chakras.
- Coworth Park introduced the Hebridean Sound Treatment in October 2023, merging traditional Hebridean sounds with guided meditation to offer a novel, restorative wellness experience.
- The World Health Organization released draft guidelines in September 2023 for the safe, effective application of traditional medicines.
- Telehealth services, expanding significantly in August 2023, have broadened the reach of SCAM, enhancing patient access to these treatments.
