causation
Psoriasis is a chronic inflammatory skin disorder, affecting the trunk and extensor surfaces of limbs and scalp predominantly. Its prevalence ranges between 0.1 and 11.4% and in India between 0.4 and 2.8%. Psoriasis remains a frequently encountered condition in homeopathy practice, but there is a dearth of evidence supporting its use. 
This 6-month, double-blind, randomized trial was conducted on 51 patients suffering from psoriasis at the National Institute of Homoeopathy, India. Patients were randomized to receive either individualized homeopathic medicines (IHMs; n = 25) in LM potencies or identical-looking placebos (n=26). Psoriasis area and severity index (PASI; primary), psoriasis disability index (PDI), and dermatological life quality index (DLQI; secondary) were measured at baseline, and every 2 months, up to 6 months. The intention-to-treat sample was analyzed using a two-way repeated measure analysis of variance.
Although intra-group changes were significant in both groups, improvements were significantly greater in the IHMs group than in the placebo group regarding the PASI scores after 6 months (F1, 49 = 10.448, P = 0.002). DLQI daily activity subscale scores also yielded similar significant results favoring IHMs against placebos after 6 months (F1, 49 = 5.480, P = 0.023). Improvement in PDI total (F1, 49 = 0.063, P = 0.803), DLQI total (F1, 49 = 1.371, P = 0.247), and all remaining subscales were higher in the IHMs group than placebos after 6 months, but non-significant statistically. Calcarea carbonica, Mercurius solubilis, Arsenicum album, and Petroleum were the most frequently prescribed medicines.
The authors concluded that IHMs exhibited better results than placebos in the treatment of psoriasis. Further research is warranted.
I am unable to access the full text of this paper [in case someone can, please send it to me for further scrutiny]. Judging from just the abstract, I see the following problems with this trial:
- Psoriasis is a genetically determined condition, and I find it hard to believe that homeopathy can change its natural history.
- The symptoms of psoriasis fluctuate and can be influenced by a range of factors, including stress.
- We learn nothing about any concomitant interventions which are always necessary, e.g. creams, or compliance with them.
- It is conceivable that patients in the verum group received inadvertent reassurance which, in turn, reduced stress and improved compliance with external treatments.
- It is unclear whether patients were successfully blinded or whether inadvertent de-blinding occurred.
In any case, I would caution that this trial needs independent replications before we can take its findings seriously.
__________________________
Thanks to several readers, I now have the full text and can add the following points:
- The authors report adverse events as follows: ” No adverse events were reported during the treatment period from either group that could be attributed causally to either IHMs or placebos. Some minor events unrelated to study medications, like common cold and injury occurring in both groups were treated with acute homeopathic medicines irrespective of allocated codes, and once those acute phases were over, the patients were returned to originally allocated groups again.” This is odd because homeopaths would expect aggravations in a high percentage of cases.
- I am not sure that I understand the blinding procedure; it is described as follows: “Double-blinding method was adopted by masking the trial participants, investigators, outcome assessors, pharmacists, and data entry operators throughout the trial. Identical-looking vials were coded as either “1” or “2” and contained either medicines or placebos. The codes remained the same for all the randomized participants. Codes were assigned randomly and confidentially by another independent third party. Both medicines and placebos were repacked in identical glass bottles and labeled with code, name of medicine, and potency, and were dispensed according to the random number list. The vials were destined for each patient by the random number chart. The participants got the medicines dispensed personally at the hospital pharmacy. Codes were broken at the end of the trial after the dataset was frozen.”
- The affiliations of the authors are interesting:1 Dept. of Materia Medica, National Institute of Homoeopathy, Ministry of AYUSH, Govt. of India, Block GE, Sector 3, Salt Lake, Kolkata 700106, West Bengal, India; affiliated to The West Bengal University of Health Sciences, Govt. of West Bengal, India
2 Dept. of Repertory, National Institute of Homoeopathy, Ministry of AYUSH, Govt. of India, Block GE,
Sector 3, Salt Lake, Kolkata 700106, West Bengal, India; affiliated to The West Bengal University of
Health Sciences, Govt. of West Bengal, India 3 Dept. of Repertory, The Calcutta Homoeopathic Medical College and Hospital, Govt. of West Bengal, 265, 266, Acharya Prafulla Chandra Road, Kolkata 700009, West Bengal; affiliated to The West Bengal University of Health Sciences, Govt. of West Bengal, India
4 East Bishnupur State Homoeopathic Dispensary, Chandi Daulatabad Block Primary Health Centre,
Village and Post Office: Dakshin Gouripur, Police Station Bishnupur, South 24 Parganas 743503, West
Bengal, under Department of Health & Family Welfare, Govt. of West Bengal, India 5 Dept. of Repertory, D. N. De Homoeopathic Medical College and Hospital, Govt. of West Bengal, 12, Gobinda Khatick Road, Tangra, Kolkata 700046, West Bengal; affiliated to The West Bengal University
of Health Sciences, Govt. of West Bengal, India.
I think it is safe to repeat that independent replications would be essential.
This study investigated the potential benefits of auricular point acupressure on cerebrovascular function and stroke prevention among adults with a high risk of stroke.
A randomized controlled study was performed with 105 adults at high risk for stroke between March and July 2021. Participants were randomly allocated to receive either
- auricular point acupressure with basic lifestyle interventions (n = 53) or
- basic lifestyle interventions alone (n = 52) for 2 weeks.
The primary outcome was the kinematic and dynamic indices of cerebrovascular function, as well as the CVHP score at week 2, measured by the Doppler ultrasonography and pressure transducer on carotids.
Of the 105 patients, 86 finished the study. At week 2, the auricular point acupressure therapy with lifestyle intervention group had higher kinematic indices, cerebrovascular hemodynamic parameters score, and lower dynamic indices than the lifestyle intervention group.
The authors concluded that ccerebrovascular function and cerebrovascular hemodynamic parameters score were greater improved among the participants undergoing auricular point acupressure combined with lifestyle interventions than lifestyle interventions alone. Hence, the auricular point acupressure can assist the stroke prevention.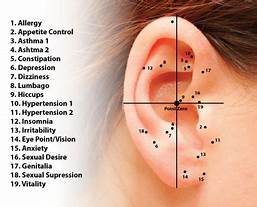
Acupuncture is a doubtful therapy.
Acupressure is even more questionable.
Ear acupressure is outright implausible.
The authors discuss that the physiological mechanism underlying the effect of APA therapy on cerebrovascular hemodynamic function is not fully understood at present. There may be two possible explanations.
- First, a previous study has demonstrated that auricular acupuncture can directly increase mean blood flow velocity in the middle cerebral artery.
- Second, cerebrovascular hemodynamic function is indirectly influenced by the effect of APA therapy on blood pressure.
I think there is a much simpler explanation: the observed effects are directly or indirectly due to placebo. As regular listeners of this blog know only too well by now, the A+B versus B study design cannot account for placebo effects. Sadly, the authors of this study hardly discuss this explanation – that’s why they had to publish their findings in just about the worst SCAM journal of them all: EBCAM.
Previous research revealed that cognitive abilities are negatively related to right-wing and prejudiced attitudes. No study has, however, investigated if emotional abilities also show such a relationship, although this can be expected based on both classic and recent literature. The aim of the present study was 2-fold:
(a) to investigate the relationship between emotional abilities and right-wing and prejudiced attitudes, and
(b) to pit the effects of emotional and cognitive abilities on these attitudes against each other.
Results from 2 adult samples (n = 409 and 574) in which abilities scores were collected in individual testing sessions, revealed that emotional abilities are significantly and negatively related to social-cultural and economic-hierarchical right-wing attitudes, as well as to blatant ethnic prejudice. These relationships were as strong as those found for cognitive abilities. For economic-hierarchical right-wing attitudes, emotional abilities were even the only significant correlate.
The authors concluded that the study of emotional abilities has the potential to significantly advance our understanding of right-wing and prejudiced attitudes.
__________
The researchers found that individuals with weaker emotional abilities — particularly emotional understanding and management — tended to score higher on a measure of right-wing authoritarianism and social dominance orientation. Right-wing authoritarianism is a personality trait that describes the tendency to submit to political authority and be hostile towards other groups, while social dominance orientation is a measure of a person’s preference for inequality among social groups.
The results of this study were univocal. People who endorse authority and strong leaders and who do not mind inequality — the two basic dimensions underlying right-wing political ideology — show lower levels of emotional abilities,” said Van Hiel, the lead author of the study. “Those with lower emotional and cognitive abilities were also more likely to agree with blatantly prejudiced statements such as “The White race is superior to all other races.”
Of course, the study only collected correlational data, preventing inferences of causality from being made. “Caution should be exercised in the interpretation of such results,” Van Hiel said. “One cannot discredit any ideology on the basis of such results as those presently obtained. Only in a distant future, we will be able to look back upon our times, and then we can maybe judge which ideologies were the best. Cognitively and emotionally smart people can make wrong decisions as well. The results have been obtained in one particular context. Would similar results be obtained in other contexts besides in a Western country with a long-standing stable democracy? Whether these tendencies are universal, or limited to particular contexts, is very intriguing.”
On this blog, we have some people who continue to promote conspiracy theories about Covid and Covid vaccinations. It is, therefore, time, I feel, to present them with some solid evidence on the subject (even though it means departing from our usual focus on SCAM).
This Cochrane review assessed the efficacy and safety of COVID‐19 vaccines (as a full primary vaccination series or a booster dose) against SARS‐CoV‐2. An impressive team of investigators searched the Cochrane COVID‐19 Study Register and the COVID‐19 L·OVE platform (last search date 5 November 2021). They also searched the WHO International Clinical Trials Registry Platform, regulatory agency websites, and Retraction Watch. They included randomized controlled trials (RCTs) comparing COVID‐19 vaccines to placebo, no vaccine, other active vaccines, or other vaccine schedules.
A total of 41 RCTs could be included and analyzed assessing 12 different vaccines, including homologous and heterologous vaccine schedules and the effect of booster doses. Thirty‐two RCTs were multicentre and five were multinational. The sample sizes of RCTs were 60 to 44,325 participants. Participants were aged: 18 years or older in 36 RCTs; 12 years or older in one RCT; 12 to 17 years in two RCTs; and three to 17 years in two RCTs. Twenty‐nine RCTs provided results for individuals aged over 60 years, and three RCTs included immunocompromised patients. No trials included pregnant women. Sixteen RCTs had two‐month follow-ups or less, 20 RCTs had two to six months, and five RCTs had greater than six to 12 months or less. Eighteen reports were based on preplanned interim analyses. The overall risk of bias was low for all outcomes in eight RCTs, while 33 had concerns for at least one outcome. 343 registered RCTs with results not yet available were identified.The evidence for mortality was generally sparse and of low or very low certainty for all WHO‐approved vaccines, except AD26.COV2.S (Janssen), which probably reduces the risk of all‐cause mortality (risk ratio (RR) 0.25, 95% CI 0.09 to 0.67; 1 RCT, 43,783 participants; high‐certainty evidence).High‐certainty evidence was found that BNT162b2 (BioNtech/Fosun Pharma/Pfizer), mRNA‐1273 (ModernaTx), ChAdOx1 (Oxford/AstraZeneca), Ad26.COV2.S, BBIBP‐CorV (Sinopharm‐Beijing), and BBV152 (Bharat Biotect) reduce the incidence of symptomatic COVID‐19 compared to placebo (vaccine efficacy (VE): BNT162b2: 97.84%, 95% CI 44.25% to 99.92%; 2 RCTs, 44,077 participants; mRNA‐1273: 93.20%, 95% CI 91.06% to 94.83%; 2 RCTs, 31,632 participants; ChAdOx1: 70.23%, 95% CI 62.10% to 76.62%; 2 RCTs, 43,390 participants; Ad26.COV2.S: 66.90%, 95% CI 59.10% to 73.40%; 1 RCT, 39,058 participants; BBIBP‐CorV: 78.10%, 95% CI 64.80% to 86.30%; 1 RCT, 25,463 participants; BBV152: 77.80%, 95% CI 65.20% to 86.40%; 1 RCT, 16,973 participants).Moderate‐certainty evidence was found that NVX‐CoV2373 (Novavax) probably reduces the incidence of symptomatic COVID‐19 compared to placebo (VE 82.91%, 95% CI 50.49% to 94.10%; 3 RCTs, 42,175 participants).There is low‐certainty evidence for CoronaVac (Sinovac) for this outcome (VE 69.81%, 95% CI 12.27% to 89.61%; 2 RCTs, 19,852 participants).High‐certainty evidence was found that BNT162b2, mRNA‐1273, Ad26.COV2.S, and BBV152 result in a large reduction in the incidence of severe or critical disease due to COVID‐19 compared to placebo (VE: BNT162b2: 95.70%, 95% CI 73.90% to 99.90%; 1 RCT, 46,077 participants; mRNA‐1273: 98.20%, 95% CI 92.80% to 99.60%; 1 RCT, 28,451 participants; AD26.COV2.S: 76.30%, 95% CI 57.90% to 87.50%; 1 RCT, 39,058 participants; BBV152: 93.40%, 95% CI 57.10% to 99.80%; 1 RCT, 16,976 participants).
Moderate‐certainty evidence was found that NVX‐CoV2373 probably reduces the incidence of severe or critical COVID‐19 (VE 100.00%, 95% CI 86.99% to 100.00%; 1 RCT, 25,452 participants).
Two trials reported high efficacy of CoronaVac for severe or critical disease with wide CIs, but these results could not be pooled.
mRNA‐1273, ChAdOx1 (Oxford‐AstraZeneca)/SII‐ChAdOx1 (Serum Institute of India), Ad26.COV2.S, and BBV152 probably result in little or no difference in serious adverse events (SAEs) compared to placebo (RR: mRNA‐1273: 0.92, 95% CI 0.78 to 1.08; 2 RCTs, 34,072 participants; ChAdOx1/SII‐ChAdOx1: 0.88, 95% CI 0.72 to 1.07; 7 RCTs, 58,182 participants; Ad26.COV2.S: 0.92, 95% CI 0.69 to 1.22; 1 RCT, 43,783 participants); BBV152: 0.65, 95% CI 0.43 to 0.97; 1 RCT, 25,928 participants). In each of these, the likely absolute difference in effects was fewer than 5/1000 participants.
Evidence for SAEs is uncertain for BNT162b2, CoronaVac, BBIBP‐CorV, and NVX‐CoV2373 compared to placebo (RR: BNT162b2: 1.30, 95% CI 0.55 to 3.07; 2 RCTs, 46,107 participants; CoronaVac: 0.97, 95% CI 0.62 to 1.51; 4 RCTs, 23,139 participants; BBIBP‐CorV: 0.76, 95% CI 0.54 to 1.06; 1 RCT, 26,924 participants; NVX‐CoV2373: 0.92, 95% CI 0.74 to 1.14; 4 RCTs, 38,802 participants).
The authors’ conclusions were as follows: Compared to placebo, most vaccines reduce, or likely reduce, the proportion of participants with confirmed symptomatic COVID‐19, and for some, there is high‐certainty evidence that they reduce severe or critical disease. There is probably little or no difference between most vaccines and placebo for serious adverse events. Over 300 registered RCTs are evaluating the efficacy of COVID‐19 vaccines, and this review is updated regularly on the COVID‐NMA platform (covid-nma.com).
_____________________
As some conspiratorial loons will undoubtedly claim that this review is deeply biased; it might be relevant to add the conflicts of interest of its authors:
- Carolina Graña: none known.
- Lina Ghosn: none known.
- Theodoros Evrenoglou: none known.
- Alexander Jarde: none known.
- Silvia Minozzi: no relevant interests; Joint Co‐ordinating Editor and Method editor of the Drugs and Alcohol Group.
- Hanna Bergman: Cochrane Response – consultant; WHO – grant/contract (Cochrane Response was commissioned by the WHO to perform review tasks that contribute to this publication).
- Brian Buckley: none known.
- Katrin Probyn: Cochrane Response – consultant; WHO – consultant (Cochrane Response was commissioned to perform review tasks that contribute to this publication).
- Gemma Villanueva: Cochrane Response – employment (Cochrane Response has been commissioned by WHO to perform parts of this systematic review).
- Nicholas Henschke: Cochrane Response – consultant; WHO – consultant (Cochrane Response was commissioned by the WHO to perform review tasks that contributed to this publication).
- Hillary Bonnet: none known.
- Rouba Assi: none known.
- Sonia Menon: P95 – consultant.
- Melanie Marti: no relevant interests; Medical Officer at WHO.
- Declan Devane: Health Research Board (HRB) – grant/contract; registered nurse and registered midwife but no longer in clinical practice; Editor, Cochrane Pregnancy and Childbirth Group.
- Patrick Mallon: AstraZeneca – Advisory Board; spoken of vaccine effectiveness to media (print, online, and live); works as a consultant in a hospital that provides vaccinations; employed by St Vincent’s University Hospital.
- Jean‐Daniel Lelievre: no relevant interests; published numerous interviews in the national press on the subject of COVID vaccination; Head of the Department of Infectious Diseases and Clinical Immunology CHU Henri Mondor APHP, Créteil; WHO (IVRI‐AC): expert Vaccelarate (European project on COVID19 Vaccine): head of WP; involved with COVICOMPARE P et M Studies (APHP, INSERM) (public fundings).
- Lisa Askie: no relevant interests; Co‐convenor, Cochrane Prospective Meta‐analysis Methods Group.
- Tamara Kredo: no relevant interests; Medical Officer in an Infectious Diseases Clinic at Tygerberg Hospital, Stellenbosch University.
- Gabriel Ferrand: none known.
- Mauricia Davidson: none known.
- Carolina Riveros: no relevant interests; works as an epidemiologist.
- David Tovey: no relevant interests; Emeritus Editor in Chief, Feedback Editors for 2 Cochrane review groups.
- Joerg J Meerpohl: no relevant interests; member of the German Standing Vaccination Committee (STIKO).
- Giacomo Grasselli: Pfizer – speaking engagement.
- Gabriel Rada: none known.
- Asbjørn Hróbjartsson: no relevant interests; Cochrane Methodology Review Group Editor.
- Philippe Ravaud: no relevant interests; involved with Mariette CORIMUNO‐19 Collaborative 2021, the Ministry of Health, Programme Hospitalier de Recherche Clinique, Foundation for Medical Research, and AP‐HP Foundation.
- Anna Chaimani: none known.
- Isabelle Boutron: no relevant interests; member of Cochrane Editorial Board.
___________________________
And as some might say this analysis is not new, here are two further papers just out:
Objectives To determine the association between covid-19 vaccination types and doses with adverse outcomes of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection during the periods of delta (B.1.617.2) and omicron (B.1.1.529) variant predominance.
Design Retrospective cohort.
Setting US Veterans Affairs healthcare system.
Participants Adults (≥18 years) who are affiliated to Veterans Affairs with a first documented SARS-CoV-2 infection during the periods of delta (1 July-30 November 2021) or omicron (1 January-30 June 2022) variant predominance. The combined cohorts had a mean age of 59.4 (standard deviation 16.3) and 87% were male.
Interventions Covid-19 vaccination with mRNA vaccines (BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna)) and adenovirus vector vaccine (Ad26.COV2.S (Janssen/Johnson & Johnson)).
Main outcome measures Stay in hospital, intensive care unit admission, use of ventilation, and mortality measured 30 days after a positive test result for SARS-CoV-2.
Results In the delta period, 95 336 patients had infections with 47.6% having at least one vaccine dose, compared with 184 653 patients in the omicron period, with 72.6% vaccinated. After adjustment for patient demographic and clinical characteristics, in the delta period, two doses of the mRNA vaccines were associated with lower odds of hospital admission (adjusted odds ratio 0.41 (95% confidence interval 0.39 to 0.43)), intensive care unit admission (0.33 (0.31 to 0.36)), ventilation (0.27 (0.24 to 0.30)), and death (0.21 (0.19 to 0.23)), compared with no vaccination. In the omicron period, receipt of two mRNA doses were associated with lower odds of hospital admission (0.60 (0.57 to 0.63)), intensive care unit admission (0.57 (0.53 to 0.62)), ventilation (0.59 (0.51 to 0.67)), and death (0.43 (0.39 to 0.48)). Additionally, a third mRNA dose was associated with lower odds of all outcomes compared with two doses: hospital admission (0.65 (0.63 to 0.69)), intensive care unit admission (0.65 (0.59 to 0.70)), ventilation (0.70 (0.61 to 0.80)), and death (0.51 (0.46 to 0.57)). The Ad26.COV2.S vaccination was associated with better outcomes relative to no vaccination, but higher odds of hospital stay and intensive care unit admission than with two mRNA doses. BNT162b2 was generally associated with worse outcomes than mRNA-1273 (adjusted odds ratios between 0.97 and 1.42).
Conclusions In veterans with recent healthcare use and high occurrence of multimorbidity, vaccination was robustly associated with lower odds of 30 day morbidity and mortality compared with no vaccination among patients infected with covid-19. The vaccination type and number of doses had a significant association with outcomes.
SECOND EXAMPLE Long COVID, or complications arising from COVID-19 weeks after infection, has become a central concern for public health experts. The United States National Institutes of Health founded the RECOVER initiative to better understand long COVID. We used electronic health records available through the National COVID Cohort Collaborative to characterize the association between SARS-CoV-2 vaccination and long COVID diagnosis. Among patients with a COVID-19 infection between August 1, 2021 and January 31, 2022, we defined two cohorts using distinct definitions of long COVID—a clinical diagnosis (n = 47,404) or a previously described computational phenotype (n = 198,514)—to compare unvaccinated individuals to those with a complete vaccine series prior to infection. Evidence of long COVID was monitored through June or July of 2022, depending on patients’ data availability. We found that vaccination was consistently associated with lower odds and rates of long COVID clinical diagnosis and high-confidence computationally derived diagnosis after adjusting for sex, demographics, and medical history.
_______________________________________
There are, of course, many more articles on the subject for anyone keen to see the evidence. Sadly, I have little hope that the COVID loons will be convinced by any of them. Yet, I thought I should give it nevertheless a try.
How often do we hear that chiropractic is safe because numerous trials reported no adverse events? This systematic review tested whether there has been a change in the reporting of adverse events associated with spinal manipulation in randomized clinical trials (RCTs) since 2016.
Databases were searched from March 2016 to May 2022: MEDLINE (Ovid), Embase, CINAHL, ICL, PEDro, and Cochrane Library. Domains of interest (pertaining to adverse events) included: completeness and location of reporting; nomenclature and description; spinal location and practitioner delivering manipulation; methodological quality of the studies and details of the publishing journal. Frequencies and proportions of studies reporting on each of these domains were calculated. Univariable and multivariable logistic regression models were fitted to examine the effect of potential predictors on the likelihood of studies reporting on adverse events.
5399 records were identified by the electronic searches, of which 154 (2.9%) were included in the analysis. Of these, 94 (61.0%) reported adverse events with only 23.4% providing an explicit description of what constituted an adverse event. Reporting of adverse events in the abstract has increased (n=29, 30.9%) while reporting in the results section has decreased (n=83, 88.3%) over the past 6 years. Spinal manipulation was delivered to 7518 participants in the included studies. No serious adverse events were reported in any of these studies.
The authors concluded as follows: while the current level of reporting of adverse events associated with spinal manipulation in RCTs has increased since our 2016 publication on the same topic, the level remains low and inconsistent with established standards. As such, it is imperative for authors, journal editors and administrators of clinical trial registries to ensure there is more balanced reporting of both benefits and harms in RCTs involving spinal manipulation.
This article is clearly relevant to our discussions about adverse events after spinal manipulation. However, I find it far too uncritical. This might be due to the affiliations of some of the authors:
- Integrative Spinal Research Group, Department of Chiropractic Medicine, University Hospital Balgrist and University of Zurich, Zurich, Switzerland.
- Department of Chiropractic, Faculty of Medicine, Health and Human Sciences, Macquarie University, Sydney, New South Wales, Australia.
Interestingly, the authors stated that they have no conflict of interest. Also interesting is the fact that they do not cite our paper from 2012. I, therefore, take the liberty of doing it:
Objective: To systematically review the reporting of adverse effects in clinical trials of chiropractic manipulation.
Data sources: Six databases were searched from 2000 to July 2011. Randomised clinical trials (RCTs) were considered, if they tested chiropractic manipulations against any control intervention in human patients suffering from any type of clinical condition. The selection of studies, data extraction, and validation were performed independently by two reviewers.
Results: Sixty RCTs had been published. Twenty-nine RCTs did not mention adverse effects at all. Sixteen RCTs reported that no adverse effects had occurred. Complete information on incidence, severity, duration, frequency and method of reporting of adverse effects was included in only one RCT. Conflicts of interests were not mentioned by the majority of authors.
Conclusions: Adverse effects are poorly reported in recent RCTs of chiropractic manipulations.
In percentage terms the results are similar. What is very different is that the authors of the new paper merely lament that the level remains low and inconsistent with established standards, while we make it clear in the abstract that adverse effect reporting is poor and in the paper identify this deficit as a violation against research ethics and thus as a form of scientific misconduct.
In view of all this, let me re-phrase the last sentence of the authors’ conclusion:
it is imperative for authors, journal editors, and administrators of clinical trial registries to ensure that researchers adhere to accepted ethical standards and that scientific misconduct no longer gets published.
Following the death from cancer of a 14-year-old Carinthian girl, the Klagenfurt public prosecutor’s office has launched an investigation against the girl’s parents. In February this year, the 14-year-old was taken to a hospital in Graz, Austria, where she died a few days later from cancer. The hospital filed charges because the tumor had been treated incorrectly with so-called alternative medicine (SCAM).
Investigations are underway on suspicion of torturing or neglecting underage, younger, or defenseless persons. Currently, the accused and witnesses are being questioned. The parents’ lawyer, Alexander Todor-Kostic, stated that the accusations were without any basis and claimed that the 14-year-old girl had decided of her own free will against being treated with chemotherapy and surgery. The parents respected this, allowed her alternative treatment methods, and acted in accordance with the applicable legal situation.
The girl had developed cancer the previous year that was not detected. Instead of seeing conventional oncologists for a reliable diagnosis and effective treatments, the parents consulted private doctors. Instead of chemotherapy, radiation, and surgery, the girl had deliberately chosen “alternative treatments” herself, the lawyer stressed.
Even though the case has been reported in several Austrian papers, I did not succeed in finding further details about it. In particular, it is unclear what type of cancer the girl had been suffering from and what type of SCAMs she received.
The Austrian skeptic Christian Kreil commented: “Sugar pills in the pharmacies, homeopathic advanced training for doctors, a proliferation of energetics offering every conceivable bullshit … the dead girl is the logical result of this esoteric foolishness covered by politics and chambers.”
I am afraid that he might have a point here: as we have discussed repeatedly on this blog, Austria is currently particularly prone to misinformation about SCAM. Here are a few examples of previous blog posts on this subject:
- Austrian osteopaths seem to violate legal, ethical and moral rules and conventions
- An open letter to the President of the Austrian Medical Association aims to stop medical quackery
- Has the ‘Vienna Medical Association’ taken leave of its senses?
- When politicians become snake-oil salesmen
- Michael Frass’ research into homeopathy for cancer: “numerous breaches of scientific integrity”
- A well-known opponent of vaccination has died of COVID after self-treatment with MMS
- The case of a boy tortured to death with homeopathy
- A truly perplexing homeopath – is it time for an official investigation?
Misinformation about SCAM can be lethal. This is one of the reasons why responsible information is so very important.
Imagine you have caught a cold. You think it is not necessary to see a doctor, but you want to take something that helps your body to get better. What is your choice of remedy? There are many options provided by conventional medicine as well as by so-called alternative medicine (SCAM).
Many people opt for SCAM to address health issues or prevent diseases. Yet, the evidence for SCAMs is either lacking or controversial due to methodological weaknesses. Thus, practitioners and patients primarily rely on subjective references rather than credible evidence from systematic research.
This study investigated whether cognitive and personality factors explain the differences in belief in SCAM and homeopathy. The researchers investigated the robustness of 21 predictors when examined together to obtain insights into some key determinants of such beliefs in a sample of 599 participants (60% female, 18-81 years). A combination of predictors explained 20% of the variance in SCAM belief. These predictors were:
- ontological confusions,
- spiritual epistemology,
- agreeableness,
- death anxiety,
- gender.
Approximately 21% of the variance in belief in homeopathy was explained by the following predictors:
- ontological confusions,
- illusory pattern perception,
- need for cognitive closure,
- need for cognition,
- honesty-humility,
- death anxiety,
- gender,
- age.
The authors concluded that some of the predictors from previous research replicated whereas others did not. Demographics and certain cognitive variables seem to be key determinants associated with beliefs in SCAM and homeopathy. Those individual differences and cognitive biases might result in a different perception of the world. However, variables related to abilities did not predict the beliefs. Thus, they might not be a result of inability but rather of ignorance.
Previous studies have shown that SCAM believers tend to believe in paranormal phenomena and conspiracies. I think that, in the discussion sections of this blog, we have ample evidence for this to be true. Paranormal beliefs are usually built on a magical worldview without reasoned review, which is shared by SCAM proponents. Such beliefs advocate emotional criteria for truth instead of data and logical considerations. Another belief, namely spirituality, is closely related to paranormal beliefs and religiosity and also associated with being a SCAM user. Lindeman found that SCAM belief could be best explained by intuitive reasoning, paranormal beliefs, and ontological confusions, defined as category mistakes in which properties of living and lifeless entities are mixed.
The authors point out that their results do not replicate previous findings that showed predictive value of certain cognitive variables such as cognitive style. An explanation could be that rather inattention to accuracy than the inability to consider empirical evidence fosters the beliefs. People might simply not be aware of the absence of evidence. Another possibility is that people are aware of the absence of evidence but are reluctant to engage with it. Practitioners and patients often claim “whatever works is good” or “the main thing is that it works”. Thus, it is ignorance rather than a lack of capacity to appropriately process the evidence.
The authors of this study are well aware of the limitations of their research:
“As with most cross-sectional studies using questionnaires, our results are based on self-reports. Additionally, single items were used for measuring belief strength. Even if multi-item measures often have advantages, single items can be advantageous in terms of practical benefits, e.g., adapting to subjects’ limited attention and time resources. There are several single item measures successfully used to measure diverse concepts including attitudes. Also, the variance on those items in our sample shows that participants were able to reflect their beliefs and rank them on the scale provided. Another limitation is that the findings are based on regression analyses, which do not provide insight into causality. Thus, the relationship remains correlational. Even if our sample was broader than in many other psychological studies—it was slightly unbalanced, especially in comparison to the German population. It over-represented educated individuals which may lead to an inadequate variation of the cognitive variables if we consider the relationship between cognition and education. However, education and the cognitive variables are only weakly correlated. Thus, it can be assumed that the unbalanced sample did not affect the distribution of cognitive variables to a great extent.”
I found this acupuncture study from the Department of Oral and Maxillofacial Sciences, “Sapienza” University of Rome, Rome, Italy. As this seems to be a respectable institution, I had a look. What I found was remarkable! Let me show you the abstract in its full beauty:
Background: Pain related to Temporomandibular Disorders (TMD) is severe, negatively affecting patients’ quality of life, and often resistant to conventional treatments. Abdominal Acupuncture (AA) is known to be particularly effective for pain, especially chronic and musculoskeletal pain, but it is still poorly studied and never investigated in TMD patients. Objectives: To analyze the efficacy of AA for the treatment of patients with subacute and chronic pain related to TMD and non-responding to previous conventional therapies (occlusal splint, medications, physical therapy).
Methods: Twenty-eight patients, 24 F and four M (mean age 49.36 years), were recruited from January 2019-February 2021. All patients underwent AA treatment: two sessions per week for four weeks, for a total of eight sessions. At the beginning of therapy (T0) and at the end of the cycle (T1) the following data were evaluated: maximum mouth opening (MMO); cranio-facial pain related to TMD (verbal numeric scale, VNS); pain interference with normal activities and quality of life of patients (Brief Pain Inventory, BPI); oral functioning (Oral Behavior Checklist, OBC); impression of treatment effectiveness (Patients’ Global Impression of Improvement, PGI-I Scale). Statistical comparison of data before and after the AA treatment was performed by Wilcoxon’s signed-rank test (significance level p < 0.05).
Results: The MMO values were significantly improved after one cycle of AA (p = 0.0002). In addition, TMD-related pain had a statistically significant decline following AA treatment (all p < 0.001). Patients’ general activity and quality of life (BPI) were described as improved following a course of AA, with statistically significant values for all aspects considered (all p < 0.05).
Conclusion: Abdominal acupuncture resulted in effective treatment of subacute/chronic resistant pain related to TMD, capable of improving mandibular function and facial pain, and reduced the interference of pain affecting patients’ quality of life.
_____________________
Shocked?
Me too!
This study did not include a control group. Such uncontrolled studies are not necessarily useless. In areas where there is no prior evidence, they can be a reasonable starting point for further research. In the case of TMD/acupuncture, however, this does not imply. Here we already have about a dozen controlled trials. This means an uncontrolled study cannot possibly contribute to our knowledge. This means that the present study is useless. And that, in turn, means it is unethical.
But even if we ignore all this, the study is very misleading. It concludes that acupuncture improved TMD. This, however, can be doubted!
- What about placebo?
- What about regression toward the mean?
- What about the natural history of the condition?
Bad science is regrettable and dangerous, as it
- wastes resources,
- misleads vulnerable patients,
- violates ethics,
- and undermines trust in science.
I fear that the Italian group has just provided us with a prime example of these points.
The claim that homeopathy has a role in oncology does not seem to go away. Some enthusiasts say it can be used as a causal therapy, while others insist it might be a helpful symptomatic adjuvant. Almost all oncologists agree that homeopathy has no place at all in cancer care.
Who is right?
This systematic review included clinical studies from 1800 until 2020 to evaluate evidence of the effectiveness of homeopathy on physical and mental conditions in patients during oncological treatment.
In February 2021 a systematic search was conducted searching five electronic databases (Embase, Cochrane, PsychInfo, CINAHL and Medline) to find studies concerning use, effectiveness, and potential harm of homeopathy in cancer patients.
From all 1352 search results, 18 studies with 2016 patients were included in this SR. The patients treated with homeopathy were mainly diagnosed with breast cancer. The therapy concepts included single and combination homeopathic remedies (used systemically or as mouth rinses) of various dilutions. The outcomes assessed were:
- the influence on toxicity of cancer treatment (mostly hot flashes and menopausal symptoms),
- the time to drain removal in breast cancer patients after mastectomy,
- survival,
- quality of life,
- global health,
- subjective well-being,
- anxiety and depression,
- safety and tolerance.
The included studies reported heterogeneous results: some studies described significant differences in quality of life or toxicity of cancer treatment favoring homeopathy, whereas others did not find an effect or reported significant differences to the disadvantage of homeopathy or side effects caused by homeopathy. The majority of the studies had low methodological quality.
The authors concluded that, the results for the effectiveness of homeopathy in cancer patients are heterogeneous, mostly not significant and fail to show an advantage of homeopathy over other active or passive comparison groups. No evidence can be provided that homeopathy exceeds the placebo effect. Furthermore, the majority of the included studies shows numerous and severe methodological weaknesses leading to a high level of bias and are consequently hardly reliable. Therefore, based on the findings of this SR, no evidence for positive effectiveness of homeopathy can be verified.
This could not be clearer. Some might argue that, of course, homeopathy cannot change the natural history of cancer, but it might improve the quality of life of those patients who believe in it via a placebo response. I would still oppose this notion: there are many effective treatments in the supportive treatment of cancer, and it seems much better to use those options and tell patients the truth about homeopathy.
A “null field” is a scientific field where there is nothing to discover and where observed associations are thus expected to simply reflect the magnitude of bias.
This analysis aimed to characterize a null field using a known example, homeopathy (a pseudoscientific medical approach based on using highly diluted substances), as a prototype. The researchers identified 50 randomized placebo-controlled trials of homeopathy interventions from highly cited meta-analyses. The primary outcome variable was the observed effect size in the studies. Variables related to study quality or impact were also extracted.
The mean effect size for homeopathy was 0.36 standard deviations (Hedges’ g; 95% confidence interval: 0.21, 0.51) better than placebo, which corresponds to an odds ratio of 1.94 (95% CI: 1.69, 2.23) in favor of homeopathy. 80% of studies had positive effect sizes (favoring homeopathy). The effect size was significantly correlated with citation counts from journals in the directory of open-access journals and CiteWatch. We identified common statistical errors in 25 studies.
The authors concluded that a null field like homeopathy can exhibit large effect sizes, high rates of favorable results, and high citation impact in the published scientific literature. Null fields may represent a useful negative control for the scientific process.
The paper is perhaps not the easiest to comprehend but once you got the idea, you will agree with me that it is BRILLIANT. I warmly recommend it to all fans of homeopathy – in fact, if I could I’d offer it to King Charles as a present for the coronation.
Its authors are among the most prominent medical epidemiologist of our time with affiliations that speak for themselves:
- Department of Epidemiology and Population Health, Stanford University, Stanford, CA, USA; Meta-Research Innovation Center at Stanford (METRICS), Stanford University, Stanford, CA, USA.
- 2Department of Epidemiology and Population Health, Stanford University, Stanford, CA, USA.
- 3Department of Epidemiology and Population Health, Stanford University, Stanford, CA, USA; Meta-Research Innovation Center at Stanford (METRICS), Stanford University, Stanford, CA, USA; Department of Medicine, Stanford University, Stanford, CA, USA; Department of Biomedical Data Science, Stanford University, Stanford, CA, USA; Department of Statistics, Stanford University, Stanford, CA, USA.
It is, of course, a pity that the article is behind a paywall – but fortunately, the senior author, John Ioannidis, published his email address together with the abstract: [email protected]. So, if you have trouble understanding the point of the analysis, I suggest you ask for a reprint to get your head around it. I promise it’s worth it.

